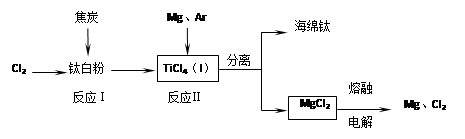��Ŀ����
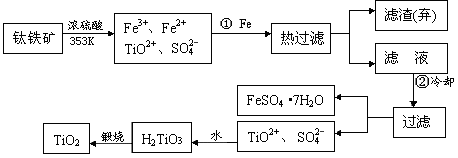
�Խ����Ͼ�������ͨ��������ά�м���һ�㱡�������������ѡ�����Fe2O3����������Ҫ�ɷ�ΪFeTiO3����ȡ����TiO2���������£�
��1��Ti��ԭ������Ϊ22��Tiλ��Ԫ�����ڱ��еĵ�________���ڣ���______�塣
��2������ټ�����Ŀ����_________________���������ȴ��Ŀ����_______________��
��3�������Ʊ��������ѵĹ����У��������õĸ�������______________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����___________________������
��4���ɽ��ʯ�Ʊ������ѣ��漰���IJ���Ϊ��
TiO2 TiCl4
TiCl4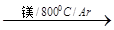 Ti
Ti
��֪����C(s)+O2(g)����CO2(g) ��H�� -393.5kJ��mol-1
��2CO(g)+O2(g)����2CO2(g) ��H�� -5665kJ��mol-1
��TiO2(s)+2Cl2(g)����TiCl4(s)+O2(g) ��H�� +41kJ��mol-1
��TiO2(s)+ 2Cl2(g)+C(s)����TiCl4(s)+2CO(g)����H��____________��
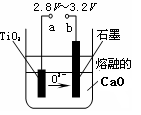
��ӦTiCl4+2Mg ���� 2MgCl2+Ti ��������н��е�������________________________��

��1��Ti��ԭ������Ϊ22��Tiλ��Ԫ�����ڱ��еĵ�________���ڣ���______�塣
��2������ټ�����Ŀ����_________________���������ȴ��Ŀ����_______________��
��3�������Ʊ��������ѵĹ����У��������õĸ�������______________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����___________________������
��4���ɽ��ʯ�Ʊ������ѣ��漰���IJ���Ϊ��
TiO2
 TiCl4
TiCl4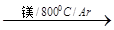 Ti
Ti ��֪����C(s)+O2(g)����CO2(g) ��H�� -393.5kJ��mol-1
��2CO(g)+O2(g)����2CO2(g) ��H�� -5665kJ��mol-1
��TiO2(s)+2Cl2(g)����TiCl4(s)+O2(g) ��H�� +41kJ��mol-1
��TiO2(s)+ 2Cl2(g)+C(s)����TiCl4(s)+2CO(g)����H��____________��
��ӦTiCl4+2Mg ���� 2MgCl2+Ti ��������н��е�������________________________��
��1��4��IVB
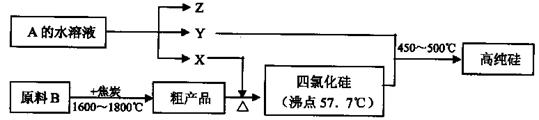
��2����Fe3+��ԭΪFe2+������������롢��õ���FeSO4��7H2O
��3��FeSO4��7H2O��ʯ�ң���̼��ơ��ϼ
��4����80kJ��mol-1����ֹ������þ������������������������̼������������
��2����Fe3+��ԭΪFe2+������������롢��õ���FeSO4��7H2O
��3��FeSO4��7H2O��ʯ�ң���̼��ơ��ϼ
��4����80kJ��mol-1����ֹ������þ������������������������̼������������
�ÿ�ͼ��ʽ������ҵ�������̣��ÿ�������Լ��۲��ͼ����ȡ������Ϣ������⣬���������������¿γ̸ĸ�ķ���������һ���Ѷȣ�����������Ϣ��������Ϣ���ۺϷ���������
��1�����Ը��ݸ�Ԫ�ص�λ���ƶ���Ԫ�ص�λ�ã���Ԫ��λ�����ڱ���������IIA���壬����λ��IVB��
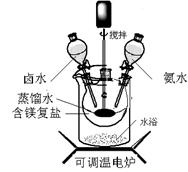
��2���۲��������ͼ��֪�����ò�Ʒ��FeSO4��7H2O��TiO2���֡����ڼ�Ũ�����������Һ����Fe3+�����Բ�����м�������Ϊ�˽�Fe3+ת����Fe2+���������ȴ��Ϊ�˽��������������ܽ�ȣ�ʹ���������ᾧ������
��3�������̵���ҪĿ������ȡ�������ѣ���������Ҫ��Ʒ����FeSO4��7H2O�Ǹ���Ʒ����Һ����ʱ���������̷�����Һ���ܻ�ʣ�ಿ���ᣬ�ʿ��Կ��Ǽ���ʯ�һ������ϼ�Խ����к͵���
��4�����ø��ڶ��ɿɼ������Ӧ�ȡ����ڸ��������½���þ��������������̼�����巢����Ӧ������Ӧ�������������þ��
��1�����Ը��ݸ�Ԫ�ص�λ���ƶ���Ԫ�ص�λ�ã���Ԫ��λ�����ڱ���������IIA���壬����λ��IVB��
��2���۲��������ͼ��֪�����ò�Ʒ��FeSO4��7H2O��TiO2���֡����ڼ�Ũ�����������Һ����Fe3+�����Բ�����м�������Ϊ�˽�Fe3+ת����Fe2+���������ȴ��Ϊ�˽��������������ܽ�ȣ�ʹ���������ᾧ������
��3�������̵���ҪĿ������ȡ�������ѣ���������Ҫ��Ʒ����FeSO4��7H2O�Ǹ���Ʒ����Һ����ʱ���������̷�����Һ���ܻ�ʣ�ಿ���ᣬ�ʿ��Կ��Ǽ���ʯ�һ������ϼ�Խ����к͵���
��4�����ø��ڶ��ɿɼ������Ӧ�ȡ����ڸ��������½���þ��������������̼�����巢����Ӧ������Ӧ�������������þ��

��ϰ��ϵ�д�
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
�����Ŀ