��Ŀ����
�±����и���ڢ����еĻ�ѧ��Ӧ��ڢ����еĻ�ѧ��Ӧ���������õڢ����е����ӷ���ʽ��ʾ���� (����)��
| ѡ�� | �ڢ��� | �ڢ��� | �ڢ��� |
| A | ������NaOH��Һ��Ͷ����Ƭ | ������NaOH��Һ��Ͷ����Ƭ | 2Al��2OH���� 2H2O===2AlO2�� ��3H2�� |
| B | ��Fe2(SO4)3��Һ�е���NaOH��Һ | ��Fe2(SO4)3��Һ�е���Ba(OH)2��Һ | Fe3����3OH��===Fe(OH)3�� |
| C | ��NaHCO3��Һ�е���Ca(OH)2��Һ | ��NaHCO3��Һ�е���NaOH��Һ | OH����HCO3��====CO32����H2O |
| D | ��NaOH��Һ��ͨ�����CO2���� | ��NaOH��Һ��ͨ������CO2���� | CO2��OH��===HCO3�� |
��A
����

��ϰ��ϵ�д�
�����Ŀ
�����й�����������ۼ�������ӷ���ʽ����ȷ���ǣ�
| ѡ�� | ������ | ���ۼ����ӷ���ʽ |
| A | H+��Fe2+��NO3-��Cl- | ���ܴ���������ͬһ��Һ�У���Ϊ������Ӧ��Fe2+ + 2H+ = Fe3+ + H2�� |
| B | Na+��K+��HCO3-��OH- | ���ܴ���������ͬһ��Һ�У���Ϊ������Ӧ��HCO3- + OH- = H2O+ CO2�� |
| C | Ca2+��NH4+��CO32-��Cl- | �ܴ���������ͬһ��Һ�� |
| D | Na+�� NH4+��SO42-��Cl- | �ܴ���������ͬһ��Һ�� |
����״̬���Ե���Ļ�������
| A��CCl4 | B��H2SO4 | C��NaOH | D��Hg |
�ܴ��������һ��������
| A��K+��Ca2+��Cl-��NO3- | B��H+��Na+��Fe2+��MnO4- |
| C��Na+��H+��SO42-��ClO- | D��K+��AlO2-��Cl-��HCO3- |
���и���������ָ�������£��ܹ�����������ǣ�������
| A���μ�KSCN��Һ����Ѫ��ɫ����Һ�У�K+��SO42�D��Mg2+��H+ |
| B����ˮ�������c(H+)��1��10-11mol/L����ɫ��Һ�У�Al3+��Cu2+��NO3�D��SO42�D |
| C����ʹ����KI��ֽ��������ɫ��Һ�У�Na+��S2�D��K+��Br�D |
| D����ʹ��ɫʯ����ֽ������Һ�У�K+��Na+��AlO2�D��HCO3�D |
�����и���Һ�У�����һ���ܴ����������
| A��ǿ������Һ�У�K����Al3����CH3COO����SO42�� |
B��ˮ���������c(H+)=10��13mol/L����Һ��K+��HCO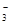 ��Br����Ba2+ ��Br����Ba2+ |
| C�������£�pH��1����ɫ��Һ�У�NH4����Mg2+��SO42����Cl- |
| D�������£�pH��1����Һ�У�Na����Fe2����NO3����SO42�� |
�������Һ��Al3+��Cu2+��Ba2+��Ag+��һ�����������Է��룬�����Լ���Cl- ��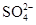 ��OH-��CO2 ��
��OH-��CO2 ��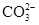 ����ȷ˳���ǣ� ��
����ȷ˳���ǣ� ��
| A���٢ڢۢ� | B���ۢݢ٢� | C���ڢ٢ۢ� | D���٢ݢۢ� |
�������ӷ���ʽ��д����ȷ����( )
A��AlCl3��Һ���ռ���Һ��Ӧ����n(OH-)��n(Al3+)=7��2ʱ�� 2Al3++7OH-=Al(OH)3��+ 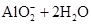 |
| B��Cl2��FeBr2��Һ��Ӧ����n(Cl2)��n(FeBr2)=1��1ʱ��2Fe2++4Br-+3Cl2=2Fe3++2Br2+6Cl- |
| C��Ca(OH)2��Һ��NaHCO3��Һ��Ӧ���� n��Ca(OH)2�ݡ�n(NaHCO3)=1��2ʱ, 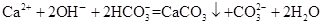 |
D��Fe��ϡ���ᷴӦ����n(Fe)��n(HNO3)=1��2ʱ��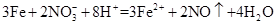 |
���н���ʵ������ķ�Ӧ����ʽ��ȷ����(����)
| A����CH3COONa��Һ��,�μӷ�̪��Һ���: CH3COO-+H2O  CH3COOH+OH- CH3COOH+OH- |
| B����H2O2��Һ��,�μ�FeCl3��Һ��������: 2H2O2+2Cl-  2H2O+O2��+Cl2�� 2H2O+O2��+Cl2�� |
| C������Ӵ���ͭƬ��пƬ����ϡ������,ͭƬ���������ݲ���: Cu+2H+  Cu2++H2�� Cu2++H2�� |
| D����Cu(OH)2����Һ�еμ�Na2S��Һ,��ɫ�������ɫ: |
 CuS(s)+2OH-
CuS(s)+2OH-