ћвƒњƒЏ»Ё
°Њћвƒњ°њ≥£ќ¬ѕ¬£ђ”–ҐўNa2CO3»№“Ї ҐЏCH3COONa»№“Ї ҐџNaOH»№“Ї Ґ№CH3COONH4»№“ЇЄч25mL£ђќп÷ µƒЅњ≈®ґ»Њщќ™0.l mol/L£ђѕ¬Ѕ–ЋµЈ®’э»Јµƒ «£® £©
A. Ћƒ÷÷»№“ЇµƒpHіу–°Ћ≥–т «Ґџ>ҐЏ>Ґў>Ґ№
B. »фЈ÷±рЉ”»л25 mL 0.1 mol/Lµƒ—ќЋб≥дЈ÷Јі”¶Їу£ђpH„оіуµƒ «Ґў
C. »фљЂЋƒ÷÷»№“Їѕ° ЌѕаЌђ±ґ э£ђpH±дїѓ„оіуµƒ «Ґ№
D. …эЄяќ¬ґ»£ђҐџ»№“ЇµƒpH≤ї±д
°Њір∞Є°њB
°Њљвќц°њA. Na2CO3°ҐCH3COONaЋЃљв≤ъ…ъOH£≠«“ЋЃљв≥ћґ»£ЇNa2CO3>CH3COONa£ђNaOHЌк»Ђµзјл≥цOH£≠£ђCH3COONH4÷–CH3COO£≠ЇЌ![]() ЋЃљв≥ћґ»ѕаЌђґш є»№“Ї≥ ÷––‘£ђЋщ“‘Ћƒ÷÷»№“ЇµƒpHіу–°Ћ≥–т «Ґџ>Ґў>ҐЏ>Ґ№£ђє Aінќу£їB. Ћƒ÷÷»№“ЇЈ÷±р«°Ї√ЈҐ…ъЈі”¶£ЇNa2CO3+HCl
ЋЃљв≥ћґ»ѕаЌђґш є»№“Ї≥ ÷––‘£ђЋщ“‘Ћƒ÷÷»№“ЇµƒpHіу–°Ћ≥–т «Ґџ>Ґў>ҐЏ>Ґ№£ђє Aінќу£їB. Ћƒ÷÷»№“ЇЈ÷±р«°Ї√ЈҐ…ъЈі”¶£ЇNa2CO3+HCl![]() NaCl+NaHCO3£ђCH3COONa+HCl
NaCl+NaHCO3£ђCH3COONa+HCl![]() CH3COOH+NaCl£ђNaOH+HCl
CH3COOH+NaCl£ђNaOH+HCl![]() NaCl+H2O£ђCH3COONH4+HCl
NaCl+H2O£ђCH3COONH4+HCl![]() CH3COOH+NH4Cl£ђNaClґ‘»№“ЇЋбЉо–‘ќё”∞ѕм£ђNaHCO3ЋЃљв є»№“Ї≥ Љо–‘£ђCH3COOH…ўЅњµзјл≤ъ…ъH+£ђNH4ClЋЃљв є»№“Ї≥ Ћб–‘£ђЋщ“‘pH„оіуµƒ «Ґў£ђє B’э»Ј£їC.ҐўҐЏҐџµƒpHЋж„≈ѕ° ЌґшЉх–°£ђЌђ ±ѕ° ЌіўљшЋЃљв£ђҐўҐЏpH±дїѓ±»Ґџ–°£ђҐ№µƒpH≤ї №ѕ° Ќ”∞ѕм£ђЋщ“‘pH±дїѓ„оіуµƒ «Ґџ£ђє Cінќу£їD. c(H£Ђ)=
CH3COOH+NH4Cl£ђNaClґ‘»№“ЇЋбЉо–‘ќё”∞ѕм£ђNaHCO3ЋЃљв є»№“Ї≥ Љо–‘£ђCH3COOH…ўЅњµзјл≤ъ…ъH+£ђNH4ClЋЃљв є»№“Ї≥ Ћб–‘£ђЋщ“‘pH„оіуµƒ «Ґў£ђє B’э»Ј£їC.ҐўҐЏҐџµƒpHЋж„≈ѕ° ЌґшЉх–°£ђЌђ ±ѕ° ЌіўљшЋЃљв£ђҐўҐЏpH±дїѓ±»Ґџ–°£ђҐ№µƒpH≤ї №ѕ° Ќ”∞ѕм£ђЋщ“‘pH±дїѓ„оіуµƒ «Ґџ£ђє Cінќу£їD. c(H£Ђ)= 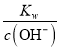 £ђ…эЄяќ¬ґ»£ђKw‘ціу£ђc(H£Ђ)‘ціу£ђҐџ»№“ЇµƒpHЉх–°£ђє Dінќу°£є —°B°£
£ђ…эЄяќ¬ґ»£ђKw‘ціу£ђc(H£Ђ)‘ціу£ђҐџ»№“ЇµƒpHЉх–°£ђє Dінќу°£є —°B°£

°Њћвƒњ°њ‘Џќ¬ґ»°Ґ»ЁїэѕаЌђµƒ3Єц√№±’»Ё∆ч÷–£ђ∞і≤їЌђЈљ љЌґ»лЈі”¶ќп£ђ±£≥÷Їгќ¬°ҐЇг»Ё£ђ≤вµ√Јі”¶іпµљ∆љЇв ±µƒ”–єЎ эЊЁ»зѕ¬°£[“—÷™N2£®g£©£Ђ3H2£®g£©![]() 2NH3£®g£©¶§H£љ£≠92.4 kJ°§mol£≠1]
2NH3£®g£©¶§H£љ£≠92.4 kJ°§mol£≠1]
»Ё∆ч | Љ„ | ““ | ±ы |
Јі”¶ќпЌґ»лЅњ | 1 mol N2°Ґ3 mol H2 | 2 mol NH3 | 4 mol NH3 |
NH3µƒ≈®ґ»£®mol°§L-1£© | c1 | c2 | c3 |
Јі”¶µƒƒ№Ѕњ±дїѓ | Ј≈≥цa kJ | ќь ’b kJ | ќь ’c kJ |
ћеѕµ—є«њ£®Pa£© | p1 | p2 | p3 |
Јі”¶ќп„™їѓ¬ | ¶Ѕ1 | ¶Ѕ2 | ¶Ѕ3 |
ѕ¬Ѕ–ЋµЈ®’э»Јµƒ «£®°°°°£©
A. 2c1£Њc3 B. ¶Ѕ1£Ђ¶Ѕ3£љ1 C. 2 p2 £Љp3 D. a£Ђb£љ92.4

