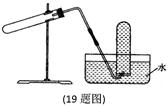
��Ŀ����
��12�֣�ijͬѧ��Ƶ���ȡ��������֤�������ֻ�ѧ���ʵ�ʵ��װ������ͼ����ʾ��
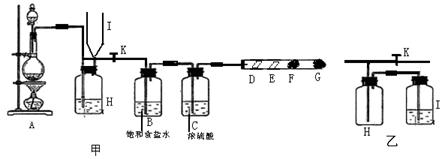
����D�����и������ɫʯ����ֽ��E������ʪ�����ɫʯ����ֽ�� F��G�����η���������������KI��Һ��ŨNaOH��Һ�����ش��������⣺
��1����װ��A�з����й�ҩƷ����K������Һ©���е�Һ�������ƿ�У��رշ�Һ©���Ļ�������ȼ�ƾ��ƣ���д��A����Ӧ�����ӷ���ʽ��
��2��B������ʳ��ˮ��Ϊ�˳�ȥ ��C��Ũ����������� ��
��3��ʵ���пɹ۲쵽��ɫ�ı仯��D�� ��E�� ��F�� ��G������������� ��G����Ӧ�����ӷ���ʽ ��
��4����F�������ɫ�������رջ���K���ɿ���Iƿ��Һ��������Hƿ�г�������ɫ���塣��Hƿ�м����Һ��һ��Ϊ ������H��Iװ�õ�����Ϊ ���������е�H��Iװ���û�Ϊ��װ�ã��Ƿ���У���ǡ��� �������� ��

����D�����и������ɫʯ����ֽ��E������ʪ�����ɫʯ����ֽ�� F��G�����η���������������KI��Һ��ŨNaOH��Һ�����ش��������⣺
��1����װ��A�з����й�ҩƷ����K������Һ©���е�Һ�������ƿ�У��رշ�Һ©���Ļ�������ȼ�ƾ��ƣ���д��A����Ӧ�����ӷ���ʽ��
��2��B������ʳ��ˮ��Ϊ�˳�ȥ ��C��Ũ����������� ��
��3��ʵ���пɹ۲쵽��ɫ�ı仯��D�� ��E�� ��F�� ��G������������� ��G����Ӧ�����ӷ���ʽ ��
��4����F�������ɫ�������رջ���K���ɿ���Iƿ��Һ��������Hƿ�г�������ɫ���塣��Hƿ�м����Һ��һ��Ϊ ������H��Iװ�õ�����Ϊ ���������е�H��Iװ���û�Ϊ��װ�ã��Ƿ���У���ǡ��� �������� ��
��1��MnO2 + 4H+ + 2Cl�� ="==" Mn2+ + Cl2�� + 2H2O
��2����ȥ�����е��Ȼ������壻��������
��3����Ϊ��ɫ��E���ȱ�����ɫ��F���������ɫ��G����������������ն����������Cl2 + 2OH��="=" Cl��+ ClO��+H2O
��4��ˮ���ռ����������������Ϊ��װ����һ���ܷ�װ�ã�������ܸı䣬ѹǿ����ʱ��Σ��
��2����ȥ�����е��Ȼ������壻��������
��3����Ϊ��ɫ��E���ȱ�����ɫ��F���������ɫ��G����������������ն����������Cl2 + 2OH��="=" Cl��+ ClO��+H2O
��4��ˮ���ռ����������������Ϊ��װ����һ���ܷ�װ�ã�������ܸı䣬ѹǿ����ʱ��Σ��
��

��ϰ��ϵ�д�
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
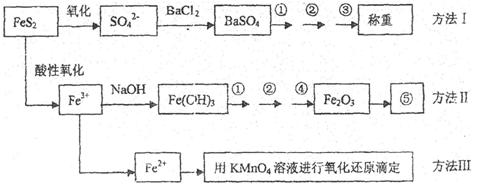
�����Ŀ
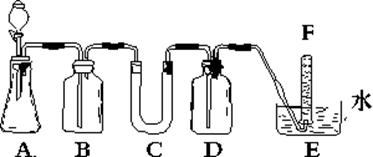
 �����������ۣ���������ȩ��
�����������ۣ���������ȩ�� ij±��������
ij±��������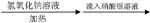 ���յij������ǰ�ɫ��
���յij������ǰ�ɫ�� ��Һ
��Һ ð�Ű���
ð�Ű��� �����������̣�
�����������̣� �ʻ�ɫ�����ۣ�����Һһ��������Ԫ��
�ʻ�ɫ�����ۣ�����Һһ��������Ԫ��