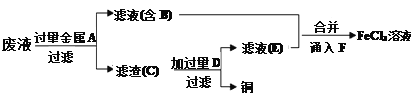
��Ŀ����
����Ŀ��������������������ڽ�������Ӧ���Ʊ�SnCl4��ij��ѧС���ͬѧ���������ʵ��װ�ý����Ʊ���
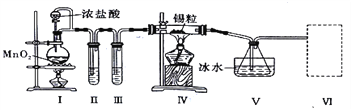
��֪���ٽ������۵�Ϊ231�棬��ѧ�������������ƣ���SnCl4�ķе�Ϊ114�棻��SnCl4����ˮ��Ӧ��
�������ͼװ�ûش�:
��1���Թ�II�е��Լ���________���Թ�III�е��Լ���____________��
��2��װ��V��������____________��
��3���b�â� ���ѡ������װ���е�________(����)��
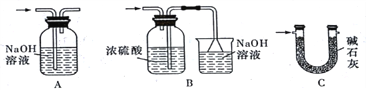
��4��ʵ�����������������װ�â���δ��Ӧ���MnO2����Ҫ�IJ���������_________��
���𰸡� ����ʳ��ˮ Ũ���� ����(�ռ�)SnCl4 C ©�������������ձ�
����������1�����ɵ������к����Ȼ����ˮ���������ȳ�ȥ�Ȼ��⣬���ñ���ʳ��ˮ��Ȼ������Ũ����������������Թ�II�е��Լ��DZ���ʳ��ˮ���Թ�III�е��Լ���Ũ���ᡣ��2��SnCl4�ķе�Ϊ114�棬���װ��V������������(�ռ�)SnCl4����3�������ж�����Ҫβ������������SnCl4����ˮ��Ӧ����Ҫ��ֹ�����е�ˮ�������룬�����b�â����Լ����ѡ�ü�ʯ�ң���ѡC����4���������̲�����ˮ��ʵ�����������������װ�â���δ��Ӧ���MnO2����Ҫ���ˣ���Ҫ�IJ���������©�������������ձ���

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����á���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѡ�
��ش��������⣺
��1��ú����������Ҫ��ѧ��Ӧ����ʽΪ��___________________________��
��2��ú�����������в������к�����H2S��Na2CO3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��________________________________________��
��3������ˮú���ϳɶ����ѵ�������Ӧ���£�
�� 2H2(g) + CO(g) ![]() CH3OH(g)����H �� ��90.8 kJ��mol��1
CH3OH(g)����H �� ��90.8 kJ��mol��1
�� 2CH3OH(g) ![]() CH3OCH3(g) + H2O(g)����H�� ��23.5 kJ��mol��1
CH3OCH3(g) + H2O(g)����H�� ��23.5 kJ��mol��1
�� CO(g) + H2O(g) ![]() CO2(g) + H2(g)����H�� ��41.3 kJ��mol��1
CO2(g) + H2(g)����H�� ��41.3 kJ��mol��1
�ܷ�Ӧ��3H2(g) + 3CO(g) ![]() CH3OCH3(g) + CO2 (g)����H�� ___________��
CH3OCH3(g) + CO2 (g)����H�� ___________��
һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��__________������ĸ���ţ�
a�����¸�ѹ b��������� c������CO2��Ũ��
d������CO��Ũ�� e�������������
��4����֪��Ӧ��2CH3OH(g) ![]() CH3OCH3(g) + H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400 �����¶��£����ܱ������м���CH3OH ����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g) + H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400 �����¶��£����ܱ������м���CH3OH ����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/��mol��L��1�� | 0.44 | 0.6 | 0.6 |
�� �Ƚϴ�ʱ�����淴Ӧ���ʵĴ�С��v�� ______ v�� ������>������<����������)��
�� ������CH3OH��10 min��Ӧ�ﵽƽ�⣬��ʱc(CH3OH) �� _________����ʱ���ڷ�Ӧ����v(CH3OH) �� __________���ﵽƽ��ʱ�״���ת��������CH3OH��= _________