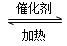
ΧβΡΩΡΎ»ί
œ¬Ν––π ω’ΐ»ΖΒΡ «(ΓΓΓΓ)
A.≈®Ε»ΨυΈΣ0.1 molΓΛ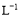 ΒΡCH3COOHΚΆCH3COONa»ή“ΚΒ»ΧεΜΐΜλΚœΥυΒΟΒΡ»ή“Κ÷–:
ΒΡCH3COOHΚΆCH3COONa»ή“ΚΒ»ΧεΜΐΜλΚœΥυΒΟΒΡ»ή“Κ÷–:
c(CH3COOH)+c(CH3COO-)=0.2 molΓΛ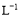
B.0.1 molΓΛ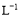 NaHCO3»ή“Κ÷–:c(Na+)=c(HC
NaHCO3»ή“Κ÷–:c(Na+)=c(HC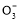 )+c(H2CO3)+2c(C
)+c(H2CO3)+2c(C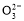 )
)
C.0.2 molΓΛ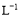 HCl»ή“Κ”κΒ»ΧεΜΐΒΡ0.1 molΓΛ
HCl»ή“Κ”κΒ»ΧεΜΐΒΡ0.1 molΓΛ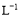 NaOH»ή“ΚΜλΚœΚσ,»ή“ΚΒΡpH=1
NaOH»ή“ΚΜλΚœΚσ,»ή“ΚΒΡpH=1
D.0.1 molΓΛ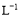 Α±Υ°÷–ΒΈ»κ0.1 molΓΛ
Α±Υ°÷–ΒΈ»κ0.1 molΓΛ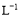 ―ΈΥα÷Ν»ή“Κ≥ ÷––‘ ±,ΜλΚœ»ή“Κ÷–:c(N
―ΈΥα÷Ν»ή“Κ≥ ÷––‘ ±,ΜλΚœ»ή“Κ÷–:c(N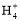 )=c(Cl-)
)=c(Cl-)
D
ΓΨΫβΈωΓΩAœν,ΝΫ÷÷»ή“ΚΒ»ΧεΜΐΜλΚœ,≈®Ε»Φθ–Γ,‘ρΗυΨί‘ΣΥΊ ΊΚψ,c(CH3COOH)+c(CH3COO-)=0.1 molΓΛ ,¥μΈσ;Bœν,”…ΈοΝœ ΊΚψΩ…÷Σ:c(Na+)=c(HC
,¥μΈσ;Bœν,”…ΈοΝœ ΊΚψΩ…÷Σ:c(Na+)=c(HC )+c(H2CO3)+c(C
)+c(H2CO3)+c(C ),¥μΈσ;Cœν,Νν»ή“ΚΧεΜΐΨυΈΣ1 L,‘ρΖ¥”Π÷°Κσc(H+)=0.05 molΓΛ
),¥μΈσ;Cœν,Νν»ή“ΚΧεΜΐΨυΈΣ1 L,‘ρΖ¥”Π÷°Κσc(H+)=0.05 molΓΛ ,¥μΈσ;Dœν,ΗυΨίΒγΚ… ΊΚψΩ…ΒΟ:c(N
,¥μΈσ;Dœν,ΗυΨίΒγΚ… ΊΚψΩ…ΒΟ:c(N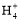 )+c(H+)=c(Cl-)+c(OH-),”…”Ύ»ή“Κ≥ ÷––‘,Υυ“‘c(H+)=c(OH-),‘ρ”–c(N
)+c(H+)=c(Cl-)+c(OH-),”…”Ύ»ή“Κ≥ ÷––‘,Υυ“‘c(H+)=c(OH-),‘ρ”–c(N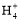 )=c(Cl-)ΓΘ
)=c(Cl-)ΓΘ

 »ΪΡή≤βΩΊ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
»ΪΡή≤βΩΊ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗœ¬±μ «Έε÷÷“χ―ΈΒΡ»ήΕ»Μΐ≥Θ ΐ(25 Γφ):
Μ·―ß Ϋ | AgCl | Ag2SO4 | Ag2S | AgBr | AgI |
»ήΕ»Μΐ | 1.8ΓΝ10-10 | 1.4ΓΝ10-5 | 6.3ΓΝ10-50 | 7.7ΓΝ10-13 | 8.51ΓΝ10-16 |
œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «(ΓΓΓΓ)
A.Έε÷÷Έο÷ ‘Ύ≥ΘΈ¬œ¬Ag2SO4±ΞΚΆ»ή“Κ÷–c(Ag+)Ήν¥σ
B.ΫΪ¬»Μ·“χ»ήΫβ”ΎΥ°Κσ,œρΤδ÷–Φ”»κNa2S,‘ρΩ…“‘…ζ≥…ΚΎ…Ϊ≥ΝΒμ
C.Ε‘”Ύ¬»Μ·“χΓΔδεΜ·“χΚΆΒβΜ·“χ»ΐ÷÷Έο÷ ‘Ύ≥ΘΈ¬œ¬ΒΡ±ΞΚΆ»ή“Κ÷–c(Ag+)ΥφΉ≈¬»ΓΔδεΓΔΒβΒΡΥ≥–ρ‘ω¥σ
D.≥ΝΒμ»ήΫβΤΫΚβΒΡΫ®ΝΔ «”–ΧθΦΰΒΡ,ΆβΫγΧθΦΰΗΡ±δ ±,ΤΫΚβ“≤ΜαΖΔ…ζ“ΤΕ·
Θ®1Θ©ΫΪΒ»Έο÷ ΒΡΝΩΒΡKIΚΆCuCl2»ή”ΎΥ°Θ§”ΟΕη–‘ΒγΦΪΒγΫβΘ§ΗΟΒγΫβΖ¥”ΠΩ…Ζ÷ΈΣ________ΗωΫΉΕΈΘ®±μΗώ≤Μ“ΜΕ®Χν¬ζΘ§»τ≤ΜΙΜΜΙΩ…“‘Ή‘––ΧμΦ”Θ©ΓΘ
ΫΉΕΈ | œύΒ±”ΎΒγΫβ ≤Ο¥»ή“Κ | άκΉ”ΖΫ≥Χ Ϋ |
ΔΌ |
|
|
ΔΎ |
|
|
Δέ |
|
|
Δή |
|
|
Δί |
|
|
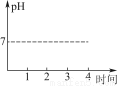
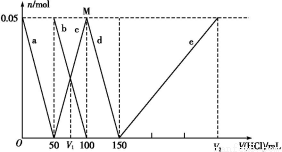
Θ®2Θ©Μ≠≥ωΙΐ≥Χ÷–»ή“ΚpHΥφ ±Φδ±δΜ·ΒΡ«ζœΏΘ®ΦΌΕ®…ζ≥…ΒΡCl2»Ϊ≤Ω“ί≥ωΘ©ΓΘ