��Ŀ����
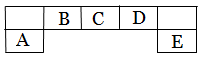
 ����������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ������ͼ��ʾ������AΪ�ؿ��к�����ߵĽ���Ԫ�ء�
����������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ������ͼ��ʾ������AΪ�ؿ��к�����ߵĽ���Ԫ�ء�
���û�ѧ����ش��������⣺
 ��1��DԪ�������ڱ��е�λ�ã�
��1��DԪ�������ڱ��е�λ�ã� ��2��A��D ��EԪ�ؼ����Ӱ뾶�ɴ�С��˳��Ϊ_____��______ ��______ (�������� )
 ��3��F��Dͬ���������ڣ�����̬�⻯���ȶ��ԵĴ�С �� ���������ţ�
��3��F��Dͬ���������ڣ�����̬�⻯���ȶ��ԵĴ�С �� ���������ţ���4���ø����������京��10���ӵ�DԪ���⻯�����ʱ��һ���������ͷ�һ�����ӣ�ͬʱ����һ�־��нϸ������Ե������ӣ���д���������ӵĵ���ʽ �����������д��ڵĻ�ѧ���� ��
 ��5��CԪ�صļ��⻯����EԪ�ص�����������ˮ���ﷴӦ�����ɻ�����K����K��ˮ��Һ��_____�ԣ�����ԡ��������ԡ������ԡ����������ӷ���ʽ��ʾ��ԭ�� ��
��5��CԪ�صļ��⻯����EԪ�ص�����������ˮ���ﷴӦ�����ɻ�����K����K��ˮ��Һ��_____�ԣ�����ԡ��������ԡ������ԡ����������ӷ���ʽ��ʾ��ԭ�� ����6��
 ������AC�����Ժã�������ϵ��С�������õ����ȳ�����ϡ������Ʊ�AC��һ�ַ���Ϊ����AԪ�ص��������̿��C�ĵ�����1600 ~ 1750������AC��ÿ����1 mol AC������18 g̼������b kJ������������������Ϊ25�桢101��3 kPa�����£�д���÷�Ӧ���Ȼ�ѧ����ʽ ��
������AC�����Ժã�������ϵ��С�������õ����ȳ�����ϡ������Ʊ�AC��һ�ַ���Ϊ����AԪ�ص��������̿��C�ĵ�����1600 ~ 1750������AC��ÿ����1 mol AC������18 g̼������b kJ������������������Ϊ25�桢101��3 kPa�����£�д���÷�Ӧ���Ȼ�ѧ����ʽ ����7����Fe��Cu�Ļ�����м���һ����C������������ˮ����ϡ��Һ����ַ�Ӧ��ʣ�����m1 g���������м���ϡ���ᣬ��ַ�Ӧ����ʣ�� m2 g ������˵����ȷ���� ��
a������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Cu2+
b������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Fe2+
c��m1һ������m2
d��ʣ�����m1 g ��һ���е���ͭ��ʣ�����m2 g ��һ��û�е���ͭ
��1���ڶ����ڵڢ�A�壨1�֣�
��2��Cl-> O2-> Al3+��2�֣�
 ��3��H2O > H2S��1�֣�
��3��H2O > H2S��1�֣�
��4��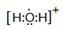 ��1�֣� ���Թ��ۼ���1�֣�
��1�֣� ���Թ��ۼ���1�֣�
��5�����ԣ�1�֣� NH4+ + H2O NH3��H2O + H+��2�֣�
NH3��H2O + H+��2�֣�
 ��6��3C(s) + Al2O3(s) + N2 (g) =" 2AlN(s)" + 3CO(g) ��H=" +2b" kJ/mol��3�֣�
��6��3C(s) + Al2O3(s) + N2 (g) =" 2AlN(s)" + 3CO(g) ��H=" +2b" kJ/mol��3�֣�
��7��b��c��2�֣�
��2��Cl-> O2-> Al3+��2�֣�
 ��3��H2O > H2S��1�֣�
��3��H2O > H2S��1�֣���4��
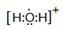 ��1�֣� ���Թ��ۼ���1�֣�
��1�֣� ���Թ��ۼ���1�֣���5�����ԣ�1�֣� NH4+ + H2O
 NH3��H2O + H+��2�֣�
NH3��H2O + H+��2�֣� ��6��3C(s) + Al2O3(s) + N2 (g) =" 2AlN(s)" + 3CO(g) ��H=" +2b" kJ/mol��3�֣�
��6��3C(s) + Al2O3(s) + N2 (g) =" 2AlN(s)" + 3CO(g) ��H=" +2b" kJ/mol��3�֣���7��b��c��2�֣�
�����������������֪��AΪ�ؿ��к�����ߵĽ���Ԫ�أ���AΪ��Ԫ�أ���ϳ���Ԫ����Ԫ�����ڱ��е�λ���жϣ�BΪ̼Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�ء�
 ��1��DΪ��Ԫ�أ������ڱ��е�λ�ã��ڶ����ڵڢ�A�壻��2��AΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�أ��������뾶�Ƚ�ԭ���жϣ������Ӱ뾶�ɴ�С��˳��ΪCl-> O2-> Al3+��
��1��DΪ��Ԫ�أ������ڱ��е�λ�ã��ڶ����ڵڢ�A�壻��2��AΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�أ��������뾶�Ƚ�ԭ���жϣ������Ӱ뾶�ɴ�С��˳��ΪCl-> O2-> Al3+�� ��3��F����ͬ���������ڣ���FΪ��Ԫ�أ�ͬ�������ϵ�����̬�⻯����ȶ�������������̬�⻯���ȶ��ԵĴ�СH2O > H2S����4������10���ӵ�DԪ���⻯�����Ϊˮ���ӣ��ø�����������ˮ����ʱ��һ���������ͷ�һ�����ӣ�ͬʱ����һ�־��нϸ������Ե������ӣ��������ӵĵ���ʽ���𰸣����������д��ڵĻ�ѧ���м��Թ��ۼ���
��3��F����ͬ���������ڣ���FΪ��Ԫ�أ�ͬ�������ϵ�����̬�⻯����ȶ�������������̬�⻯���ȶ��ԵĴ�СH2O > H2S����4������10���ӵ�DԪ���⻯�����Ϊˮ���ӣ��ø�����������ˮ����ʱ��һ���������ͷ�һ�����ӣ�ͬʱ����һ�־��нϸ������Ե������ӣ��������ӵĵ���ʽ���𰸣����������д��ڵĻ�ѧ���м��Թ��ۼ��� ��5����Ԫ�صļ��⻯������Ԫ�ص�����������ˮ���ﷴӦ�����ɻ������Ȼ�泥�ˮ��Һ�����ԣ������ӷ���ʽ��ʾ��ԭ��NH4+ + H2O
��5����Ԫ�صļ��⻯������Ԫ�ص�����������ˮ���ﷴӦ�����ɻ������Ȼ�泥�ˮ��Һ�����ԣ������ӷ���ʽ��ʾ��ԭ��NH4+ + H2O  NH3��H2O + H+����6��
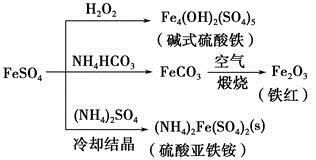
NH3��H2O + H+����6�� ��������֪������������̿�͵�����1600 ~ 1750�����ɵ�������ÿ����1 mol ������������18 g̼������b kJ������������������Ϊ25�桢101��3 kPa�����£��÷�Ӧ���Ȼ�ѧ����ʽΪ3C(s) + Al2O3(s) + N2 (g) =" 2AlN(s)" + 3CO(g) ��H=" +2b" kJ/mol����7����Fe��Cu�Ļ�����м���һ��������ϡ��Һ���������Ļ�ԭ�Ա�ͭǿ�����������ᷴӦ����ַ�Ӧ����������������������������ͭ��ʣ�����Ϊͭ������ͭ�Ļ���m1 g���������м���ϡ���ᣬ����3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O����3Fe+2NO3-+8H+=3Fe2++2NO��+4H2O��a������ϡ����ǰ�ͼ���ϡ��������Һ�ж���һ����Cu2+������b������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Fe2+����ȷ��c��m1һ������m2����ȷ��d��ʣ�����m1 g ��һ���е���ͭ��ʣ�����m2 g �п����е���ͭ������ѡbc��
��������֪������������̿�͵�����1600 ~ 1750�����ɵ�������ÿ����1 mol ������������18 g̼������b kJ������������������Ϊ25�桢101��3 kPa�����£��÷�Ӧ���Ȼ�ѧ����ʽΪ3C(s) + Al2O3(s) + N2 (g) =" 2AlN(s)" + 3CO(g) ��H=" +2b" kJ/mol����7����Fe��Cu�Ļ�����м���һ��������ϡ��Һ���������Ļ�ԭ�Ա�ͭǿ�����������ᷴӦ����ַ�Ӧ����������������������������ͭ��ʣ�����Ϊͭ������ͭ�Ļ���m1 g���������м���ϡ���ᣬ����3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O����3Fe+2NO3-+8H+=3Fe2++2NO��+4H2O��a������ϡ����ǰ�ͼ���ϡ��������Һ�ж���һ����Cu2+������b������ϡ����ǰ�ͼ���ϡ��������Һ�п϶�����Fe2+����ȷ��c��m1һ������m2����ȷ��d��ʣ�����m1 g ��һ���е���ͭ��ʣ�����m2 g �п����е���ͭ������ѡbc�� 
��ϰ��ϵ�д�
�����Ŀ


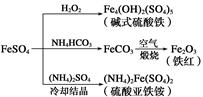
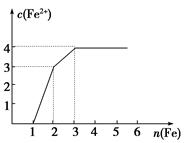
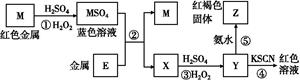
 E(SCN)3��
E(SCN)3��