��Ŀ����
����ѧ����ѡ��2����ѧ�뼼������15�֣�
ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⡣
��1���о����ֺ�ˮ�����ķ�����_________��_________��
��2���ȼҵͨ����ⱥ��ʳ��ˮ�ܻ���ռ�����������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ������ʯī�ӵ�Դ_________�������ʱ���缫�ĵ缫��ӦʽΪ_________����������ͨ�����ӽ���Ĥ����Ҫ������__________��
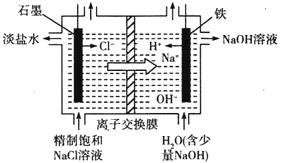
��3�������Ƽ�У�������ʳ��ˮ��ͨ��CO2��NH3�Ʊ�NaHCO3���仯ѧ����ʽΪ____________����ͨ�����__________���ѧʽ������������__________________ ��������NaHCO3���ȷֽ���Ʊ����
��4��Ŀǰ������60%���ϵ�þ���ǴӺ�ˮ����ȡ�ģ���֪��MgO��MgCl2���۵�ֱ�Ϊ2852���714�档����˵����ҵ�ϲ��õ������MgCl2�����ǵ������MgO������__________ ______________________________________________________________________ ��
ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⡣
��1���о����ֺ�ˮ�����ķ�����_________��_________��
��2���ȼҵͨ����ⱥ��ʳ��ˮ�ܻ���ռ�����������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ������ʯī�ӵ�Դ_________�������ʱ���缫�ĵ缫��ӦʽΪ_________����������ͨ�����ӽ���Ĥ����Ҫ������__________��

��3�������Ƽ�У�������ʳ��ˮ��ͨ��CO2��NH3�Ʊ�NaHCO3���仯ѧ����ʽΪ____________����ͨ�����__________���ѧʽ������������__________________ ��������NaHCO3���ȷֽ���Ʊ����
��4��Ŀǰ������60%���ϵ�þ���ǴӺ�ˮ����ȡ�ģ���֪��MgO��MgCl2���۵�ֱ�Ϊ2852���714�档����˵����ҵ�ϲ��õ������MgCl2�����ǵ������MgO������__________ ______________________________________________________________________ ��
��1���������ӽ�������������������ѡ��������2�֣�
��2��2NaCl+2H2O���2NaOH+H2��+Cl2����2�֣� ����1�֣� 2H++2e-
 H2����2�֣�
H2����2�֣� Na+��H+��1�֣�
��3��NaCl+NH3+CO2+H2O
 NaHCO3+NH4Cl��2�֣� NH3��1�֣� NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С��2�֣�
NaHCO3+NH4Cl��2�֣� NH3��1�֣� NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С��2�֣���4�������۵�MgO��MgCl2�����MgO�ķѵ�࣬�����MgCl2�ĵ���٣�2�֣�
���������
��1���������ӽ�������������������ѡ������
��2��2NaCl+2H2O���2NaOH+H2��+Cl2�� �� 2H++2e-
 H2��
H2�� Na+��H+
��3��NaCl+NH3+CO2+H2O
 NaHCO3+NH4Cl NH3 NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С
NaHCO3+NH4Cl NH3 NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С��4�������۵�MgO��MgCl2�����MgO�ķѵ�࣬�����MgCl2�ĵ����

��ϰ��ϵ�д�
�����Ŀ

 2Na +Cl2��
2Na +Cl2�� Mg+ H2O
Mg+ H2O  3 Fe +4 CO2
3 Fe +4 CO2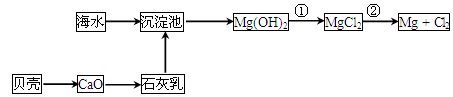


 2CuI(��ɫ)����I2��I2��2S2O32��
2CuI(��ɫ)����I2��I2��2S2O32��