��Ŀ����
����Ŀ�����ú˴Ź������ⶨ�л�����ӵ���ά�ṹ���о������2002��ŵ������ѧ�������л�������У���ͬ��ԭ�ӵĺ˴Ź������и����ķ�ֵ���ź���Ҳ��ͬ�����ݷ�ֵ���ź�������ȷ���л����������ԭ�ӵ��������Ŀ����������ѵĽṹ��ʽΪ��CH3��CH2��O��CH2��CH3
��˴Ź������и����ķ�ֵ���ź���������������ͼ��һ����ʾ��
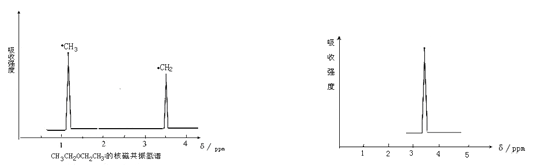
��һ�� ������
��1�����������У���˴Ź��������и����ķ�ֵ���ź���ֻ��һ������ ��
A��CH3CH3 B��CH3COOH
C�� CH3COOCH3 D��CH3OCH3
��2��������A��B�ķ���ʽ����C2H4Br2, A�ĺ˴Ź�������ͼ����ͼ��������ʾ����A�Ľṹ��ʽΪ�� ����Ԥ��B�ĺ˴Ź����������� �������ź�����������֮��Ϊ ��
��3����ij�л��ﺬ��̼������������Ԫ��,���к�̼40%,����6.67%,����л����ʵ��ʽΪ �������л���������ܶ�����ͬ״�������������ܶȵ�30��,��ȷ�����л������ʽ �������л������������Ʒ�Ӧ������̼�����Ʒ�Ӧ�����Ʋ���л���Ľṹ��ʽ ��
��ͬ���칹����Ĺ㷺����������л�����������Ҫԭ��֮һ����ϩ�ж���ͬ���칹�壬���д���˳���칹�Ľṹ����Ϊ ��
���л���CH3OH��CH3CH2OH��һ��ͬϵ�CH3OH��������Ƽ���ȼ�ϵ���������ΪKOH��Һ����д���õ�ص������缫��Ӧʽ ������Ӧ��������3mol ����ת�ƣ���Ӧ��CH3OH������Ϊ g
���𰸡���1��AD��2����4����BrCH2CH2Br��2��3��1����1��3��
��3����CH2O��C2H4O2��CH3COOH��2-��ϩ������4����O2+2H2O+4e��=4OH����16
��������
�����������1�������л�������ԭ��������жϡ�һ��ͬһ��̼ԭ���ϵ���ԭ������ͬ�ģ�ͬһ��̼ԭ�������ӵļ��ϵ���ԭ������ͬ�ģ����жԳ��Խṹ��1��A��CH3CH3��ֻ��һ��Hԭ�ӣ�A��ȷ��B��CH3COOH��������Hԭ�ӣ�B������C��CH3COOCH3��������Hԭ�ӣ�C������D��CH3OCH3��ֻ��һ��Hԭ�ӣ�D��ȷ��
��2���˴Ź���������ֻ����һ���壬˵����������ֻ��1��Hԭ�ӣ���A�Ľṹ��ʽΪBrCH2CH2Br��B�Ľṹ��ʽΪCH3CHBr2��������Hԭ�ӣ����Ժ˴Ź�����������2���壬����ΪBrCH2CH2Br��B�Ľṹ��ʽΪCH3CHBr2���еķ�Ϊ2����������֮��Ϊ3��1��
��3��N��C����N��H����N��O����40%/12:6.67%/1:��1-40%-6.67%��/16==1:2:1,ʵ��ʽ��CH2O�������л���������ܶ�����ͬ״�������������ܶȵ�30��,���л����Ħ������Ϊ30��2=60�������л������������Ʒ�Ӧ������̼�����Ʒ�Ӧ��˵�����л��ﺬ���ǻ����ֺ����Ȼ����л���Ľṹ��ʽ��CH3COOH
����ϩ�ṹ�У�1-��ϩ�ṹ��ʽCH3-CH2-CH=CH2��2-��ϩ�ṹ��ʽCH3-CH=CH-CH3������-��ϩ�ṹ����˳���칹��
��CH3OH�ڼ��Ի�������������Ӧ����̼��غ�ˮ������һ������ȼ�ϵ�أ���������������Ӧ��CH3OH- 6e��+8OH-=CO32-+6H2O; ����������ԭ��Ӧ��O2 + 2H2O + 4e��== 4OH�������ݹ�ϵʽ����ת��3Ħ�����ӣ����ļ״�0.5Ħ��������Ϊ16�ˡ�
