��Ŀ����
12����4.6gN2O4��������ݻ�Ϊ2L�������У����µ�25�棬N2O4��ȫ������������Ӧ��N2O4?2NO2����Ӧ�ﵽƽ���ͬ���²��ѹǿΪN2O4����ȫ������δ������Ӧʱѹǿ��1.2���������в���ȷ���ǣ�������| A�� | �������¶ȣ�������ɫ������ | |
| B�� | ƽ��ʱNO2��N2O4�����ʵ���֮��Ϊ1��2 | |
| C�� | ƽ��ʱ��������ƽ����Է�������ԼΪ76.7 | |
| D�� | ����ͬ�¶��µ���һ������N2O4Ũ��Ϊ0.03mol/L��NO2Ũ��Ϊ0.01mol/Lʱ����Ӧ���ʵĹ�ϵΪv����v�� |
���� 4.6gN2O4����Ϊ0.05mol��ͬ��ͬ��ʱ�������ѹǿ֮�ȵ��������ʵ���֮�ȣ����Է�Ӧ���������ʵ���Ϊ0.05mol��1.2=0.06mol������0.01molN2O4�ֽ�������0.02molNO2��ʣ��N2O40.05mol-0.01mol=0.04mol���ݴ˷�����
��� �⣺A��N2O4?2NO2�Ƿ��ȷ�Ӧ������ƽ�������ƶ������������ɫ��dz����A����
B��ƽ��ʱ��0.02molNO2��0.04molN2O4��NO2��N2O4�����ʵ���֮��Ϊ1��2����B��ȷ��
C����������ƽ����Է�������=$\frac{�������������ܺ�}{�����������ʵ����ܺ�}$=$\frac{4.6g}{0.06mol}$=76.7����C��ȷ��
D��ƽ��ʱ����0.02molNO2��0.04molN2O4��K=$\frac{[N{O}_{2}]^{2}}{[{N}_{2}{O}_{4}]}$=$\frac{0.0{2}^{2}}{0.04}$=0.01������ͬ�¶��µ���һ������N2O4Ũ��Ϊ0.03mol/L��NO2Ũ��Ϊ0.01mol/Lʱ��Qc=$\frac{0.0{1}^{2}}{0.03}$=$\frac{0.01}{3}$��K��ƽ�⽫�������ƶ�������v����v������D��ȷ��
��ѡA��
���� ���⿼���˻�ѧƽ����ƶ���ƽ����Է������������㡢��Ũ�����뻯ѧƽ�ⳣ���Ĵ�С�ж�ƽ���ƶ��ķ�����Ŀ�ѶȲ���

| A�� | ��Ϊp����ǡ�8�����εģ�����p�ĵ����ߡ�8������ | |
| B�� | K�ܼ���3s��3p��3d��3f�ĸ���� | |
| C�� | ��ԭ��ֻ��һ�����ӣ�����ԭ��ֻ��һ����� | |
| D�� | ����˵��������ȷ |
��a=b+4����a+b=8�� ��a+b=30�� ��a=b+8��
| A�� | �٢ڢۢ� | B�� | �٢ۢ� | C�� | �ڢ� | D�� | �ۢ� |
| A�� | 100 mL 0.3 mol/L��HCl��50 mL 0.3 mol/L��NaOH���� | |
| B�� | 80mL 0.3 mol/L��HCl��80 mL 0.3 mol/L��NaOH��Һ���� | |
| C�� | 80mL 0.3 mol/L��H2SO4��80 mL 0.3 mol/L��NaOH��Һ���� | |
| D�� | 50mL 0.3 mol/L��H2SO4��50 mL 0.6 mol/L��NaOH��Һ���� |
| A�� | 2-��-2-��ϩ | B�� | ���� | ||
| C�� | 2��2��3��3-�ļ����� | D�� | 3��4-�������� |
 ������������Cu2+�γ�[Cu��H2O��4]+���ѧʽ����ʹCuCl2��Һ����ɫ��
������������Cu2+�γ�[Cu��H2O��4]+���ѧʽ����ʹCuCl2��Һ����ɫ��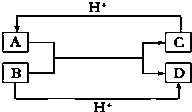
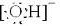 ��C�Ľṹʽ
��C�Ľṹʽ ��?
��?