��Ŀ����
15���ɶ�����Ԫ����ɵĻ�����X��ij����ҩ����Ч�ɷ֣���ͬѧ��̽��X����ɣ��������ϣ����ɶ�����Ԫ����ɵĿ���ҩ����Ч�ɷ���̼�����ơ�̼��þ����������������þ��������������ʽ̼��þ������Al3+��pH=5.0ʱ������ȫ��Mg2+��pH=8.8ʱ��ʼ��������pH=11.4ʱ������ȫ��ʵ����̣�
������X��ĩ�м���������ᣬ������ɫ��ζ����A���õ���ɫ��Һ��
���ò�˿պȡ����I�����õ���Һ���ڻ��������գ���ɫ���森
����I�����õ���Һ�еμӰ�ˮ������pH��5��6��������ɫ����B�����ˣ�
���������B�мӹ���NaOH��Һ������ȫ���ܽ⣮
��������еõ�����Һ�еμ�NaOH��Һ������pH��12���õ���ɫ����C��
��1��A���ӵĵ���ʽΪ��
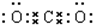 ��C�Ļ�ѧʽΪMg��OH��2
��C�Ļ�ѧʽΪMg��OH��2��2��ʵ���֮ǰҪ�Բ�˿���д��������������Dz�˿��ϴϡ���Ტ�ھƾ��ƻ����������ظ�2-3��
��3���ڢ�ʵ������������Ӧ�����ӷ���ʽ��Al3++3NH3•H20�TAl��OH��3��+3NH4+
��4������B�ܽ�����ӷ���ʽ��Al��OH��3+OH-�TAlO2-+2H2O
��5��������n��A����n��B����n��C��=1��2��3����X�Ļ�ѧʽ��Mg3Al2��OH��10CO3��
���� ������X��ĩ�м���������ᣬ������ɫ��ζ����A����Ͽ���ҩ����Ч�ɷ֣�֪������ΪCO2��
��X��һ������Na����ΪNa����ɫΪ��ɫ��
���������Ϣ֪����pH��5��6ʱ���ɵİ�ɫ����ΪAl��OH��3��
�����������NaOH��Һ������B��ȫ�ܽ⣬���ӷ���ʽΪ��Al��OH��3+OH-�TAlO2-+2H2O��
��������NaOH��Һ����pH��12���а�ɫ���������������CΪMg��OH��2��
��1��A����ΪCO2��������̼�д�������̼��˫��������CΪMg��OH��2��
��2����ɫ��Ӧ��ϡ����ϴ����˿�����ղ�˿���ٽ�����ɫ��Ӧ��
��3�������ӺͰ�ˮ��Ӧ��������������
��4�����������������ԣ����ڹ���NaOH��Һ��
��5������ԭ���غ��Լ�����غ��ƶ�X�Ļ�ѧʽ��
��� �⣺��1���������м���������ᣬ��������������̼���Ρ�̼�����κ����ᷴӦ���ɶ�����̼������X��ĩ�м���������ᣬ������ɫ��ζ����A��
������ΪCO2��������̼Ϊֱ���ͽṹ�������д�������̼��˫����������̼�ĵ���ʽΪ��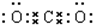 ������NaOH��Һ����pH��12���а�ɫ���������������CΪMg��OH��2��
������NaOH��Һ����pH��12���а�ɫ���������������CΪMg��OH��2��
�ʴ�Ϊ��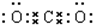 ��Mg��OH��2��
��Mg��OH��2��
��2����ɫ��Ӧ��ϡ����ϴ����˿��ȥ���ʣ���ϴ��Ϊ��ϴȥ��������Ĥ��������ϴ��֮��ͨ�����ھƾ��ƻ��������գ���Ϊ�����Ȼ����ӷ��������������Ӿ�һ���ӷ������������ǣ���˿��ϴϡ���Ტ�ھƾ��ƻ����������ظ�2-3�Σ�
�ʴ�Ϊ����˿��ϴϡ���Ტ�ھƾ��ƻ����������ظ�2-3�Σ�
��3������pH��5��6ʱ���ɵİ�ɫ����ΪAl��OH��3��NH3•H20Ϊ������ʣ����ӷ���ʽ��ӦдΪ��ѧʽ��
�ʴ�Ϊ��Al3++3NH3•H20�TAl��OH��3��+3NH4+��
��4��Al��OH��3Ϊ�����������������ǿ��������NaOH��Һ��Al��OH��3������ȫ�ܽ⣬���ӷ���ʽΪ��Al��OH��3+OH-�TAlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-�TAlO2-+2H2O��
��5������n��CO2����n[Al��OH��3]��n[Mg��OH��2]=1��2��3����CO32-��Al3+��Mg2+�����ʵ���֮��Ϊ1��2��3����ϵ���غ㣬��CO32-��Al3+��Mg2+��OH-�����ʵ���֮��Ϊ1��2��3��10����XΪMg3Al2��OH��10CO3��
�ʴ�Ϊ��Mg3Al2��OH��10CO3��
���� ���⿼�鿹��ҩ�ɷֵ�̽��ʵ�飬��Ŀ��Ϊ�ۺϣ��Ѷ��еȣ������״���Ϊ�ڣ�5���⣬���������غ㶨�ɽ��

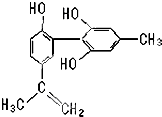 �ҹ�֧�֡����İ��ˡ���һ����Ҫ�����ǣ���������˶�Ա�����˷ܼ���ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵����ȷ���ǣ�������
�ҹ�֧�֡����İ��ˡ���һ����Ҫ�����ǣ���������˶�Ա�����˷ܼ���ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵����ȷ���ǣ�������| A�� | ��FeCl3��Һ����ɫ����Ϊ�������뱽������ͬϵ�� | |
| B�� | �÷����е�����̼ԭ�Ӳ����ܹ�ƽ�� | |
| C�� | ����KMnO4��H+����Һ���۲��Ϻ�ɫ��dz��������Ϊ�ṹ�д���̼̼˫�� | |
| D�� | 1mol�����ʷֱ���Ũ��ˮ��H2��Ӧʱ�������Br2�� H2�ֱ�Ϊ4mol��7mol |
| A�� | CO2��NO2��SO2���ڴ�����Ⱦ�� | |
| B�� | ����60Co�ķ����Կ�����ijЩ������60Co��59Co��Ϊͬλ�� | |
| C�� | ������CO2���������ӻᵼ������ЧӦ�Ӿ� | |
| D�� | ʳ�ο�����ζ����Ҳ����ʳƷ������ |
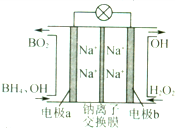 �ݱ����������⻯����NaBH4��BԪ�صĻ��ϼ�Ϊ+3�ۣ���H2O2��ԭ�ϵ�ȼ�ϵ�أ��������ϲ���Pt/C���������ϲ���MnO2���������վ�ͨ�����ǵ�Դ���䶡��ԭ����ͼ��ʾ������˵���д�����ǣ�������
�ݱ����������⻯����NaBH4��BԪ�صĻ��ϼ�Ϊ+3�ۣ���H2O2��ԭ�ϵ�ȼ�ϵ�أ��������ϲ���Pt/C���������ϲ���MnO2���������վ�ͨ�����ǵ�Դ���䶡��ԭ����ͼ��ʾ������˵���д�����ǣ�������| A�� | ��طŵ�ʱNa+��a��������b���� | |
| B�� | �缫b����Mn O2��Mn O2�����缫�������д����� | |
| C�� | �õ�صĸ�����ӦΪBH${\;}_{4}^{-}$+8OH-һ8e-�TBO${\;}_{2}^{-}$+6H2O | |
| D�� | ÿ����3molH2O2��ת�Ƶĵ���Ϊ3mol |
| A�� | AgNO3 | B�� | H2SO4 | C�� | BaCl2 | D�� | NaOH |

����˵����ȷ���ǣ�������
| A�� | ��ҺA�е�������ΪFe2+��Fe3+��H+ | B�� | ��Ʒ��FeԪ�ص�����Ϊ2.24 g | ||
| C�� | ��Ʒ��CuO������Ϊ4.0 g | D�� | V=896 mL |
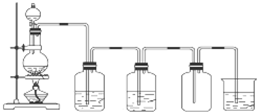 ��1��ָ��ʹ��������� ����ϴ��������Ʒ�ĵ�һ��������
��1��ָ��ʹ��������� ����ϴ��������Ʒ�ĵ�һ��������| ����-KI��ֽ�������� | ����ƿ�ռ����� | ����ƿ |
| ������ˮʪ�� | ���O��ƿ | �����Ƿ�©ˮ����©�� |
| Ӧ����NaOH������/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| 4.0 | 500 | �ձ�����Ͳ����������ҩ�ס�������ƽ����ͷ�ι� |
A������ʱ��NaOH����ֱ�ӷ���ֽ��
B����������NaOH����¶���ڿ�����ʱ�����
C��ѡ�õ�����ƿ��������������ˮ
D�����ձ����ܽ�NaOH��������������Һע������ƿ��
E���������ƹ���������ƿ����
��4������������Һ�������ͺ��Ȼ�����Һ ��39%���Ҵ���Һ ���Ȼ��ƺ͵������ˮ��Һ���������ϸ����Һ����ȷ���������Ƿ�Һ��������ȡ��Һ��
��5����ͼ��ʵ������ȡ������װ�ã���д������װ�������ԵIJ����������رշ�Һ©�����������ձ��м�ˮû�����ܿڣ���Բ����ƿ�������ܿ������ݣ���ֹͣ����ȴ���ܿ��γ��ȶ���ˮ������װ�õ����������ã���
| A�� | �����������ֱ������������������������������Һ��Ӧ�Ƶõ����������� | |
| B�� | ���Ȼ�����Һ�еμӹ�����ˮ�����յõ�������Һ | |
| C�� | ��������������������Ũ���ᷴӦ�Ƶô�����NO2 | |
| D�� | FeCl3��Һ�������ɵõ���ˮ�Ȼ������� |
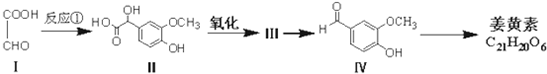
 ��
��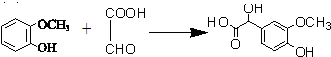 ��
��