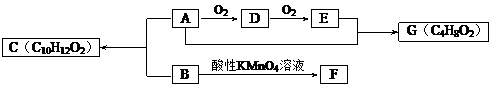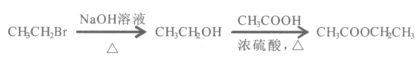��Ŀ����
����Ŀ��������һ����Ҫ������������ṹ��ʽΪ��![]() ���ڶ������ﻯѧ��������������Ҫ��������һ����³´�л���˶�������ϱ���������ش��������⣺
���ڶ������ﻯѧ��������������Ҫ��������һ����³´�л���˶�������ϱ���������ش��������⣺
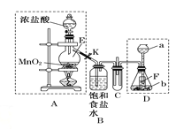
��1����ϵͳ��������������___________________��
��2�����ᷢ����ȥ��Ӧ���ò���Ľṹ��ʽΪ______________________��
��3��1mol����ֱ���������Na��Na2CO3��NaHCO3��Ӧʱ�����������ʵ����ʵ���֮��Ϊ___________��
��4��������Cu��Ag������ʱ�ᱻ����������д���˷�Ӧ�Ļ�ѧ����ʽ��
____________________________________________________________________________��
���𰸡���1��2���ǻ����� ��2��CH2��CH��COOH ��3��4:1:2
��4�� ![]()
��������
�����������1�� ������ϵͳ������������2���ǻ����ᡣ
��2�����ᷢ����ȥ��Ӧ���ò���Ľṹ��ʽΪCH2��CH��COOH��
��3��1mol����ֱ����������ǻ����Ȼ�������Na��Ӧ���Ȼ�����Na2CO3��NaHCO3��Ӧ�����������ʵ����ʵ���֮��Ϊ4:1:2��
��4��������Cu��Ag������ʱ�ᱻ������������Ӧ�Ļ�ѧ����ʽ��![]() ��
��

��ϰ��ϵ�д�
�����Ŀ