��Ŀ����
��������Ҫ�Ļ���ԭ�ϣ���ش��йذ����������
��1�����������ǹ�ҵ�������һ����Ҫת������֪��
4NH3��g����3O2��g��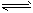 2N2��g��+6H2O��g�� ��H= -1266.8 kJ��mol-1
2N2��g��+6H2O��g�� ��H= -1266.8 kJ��mol-1
2NO��g��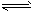 N2��g����O2��g�� ��H= - 180.5 kJ��mol-1
N2��g����O2��g�� ��H= - 180.5 kJ��mol-1
д����������4NH3+5O2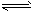 4NO+6H2O ���Ȼ�ѧ����ʽ___________________
4NO+6H2O ���Ȼ�ѧ����ʽ___________________
��2������������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=__________�÷�Ӧ��һ��������_______���ܡ����ܣ��Է���Ӧ��������________________________��
��3������ˮ�ʼ��ԣ�д����ˮ�ʼ��Ե����ӷ���ʽ___________________��0.1 mol/L��ˮ��0.1 mol��L-1����������ϣ�д���кͷ�Ӧ�����ӷ���ʽ_____________��Ӧ����Һ������Ũ���ɴ�С����Ϊ_________________��
��4���ϳɰ��У���ȡ�����ķ���֮һ�ǣ�CO(g)+H2O(g)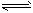 CO2(g)+H2(g) ��H<0�÷�Ӧ��427��Cʱ��ƽ�ⳣ����9�������Ӧ��ʼʱ��һ����̼��ˮ������Ũ�ȶ���0.1mol/L������һ����̼��ת���ʡ���д��������̣�
CO2(g)+H2(g) ��H<0�÷�Ӧ��427��Cʱ��ƽ�ⳣ����9�������Ӧ��ʼʱ��һ����̼��ˮ������Ũ�ȶ���0.1mol/L������һ����̼��ת���ʡ���д��������̣�
��1�����������ǹ�ҵ�������һ����Ҫת������֪��
4NH3��g����3O2��g��
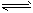 2N2��g��+6H2O��g�� ��H= -1266.8 kJ��mol-1
2N2��g��+6H2O��g�� ��H= -1266.8 kJ��mol-12NO��g��
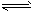 N2��g����O2��g�� ��H= - 180.5 kJ��mol-1
N2��g����O2��g�� ��H= - 180.5 kJ��mol-1 ���������4NH3+5O2
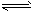 4NO+6H2O ���Ȼ�ѧ����ʽ___________________
4NO+6H2O ���Ȼ�ѧ����ʽ___________________ ��2������������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=__________�÷�Ӧ��һ��������_______���ܡ����ܣ��Է���Ӧ��������________________________��
��3������ˮ�ʼ��ԣ�д����ˮ�ʼ��Ե����ӷ���ʽ___________________��0.1 mol/L��ˮ��0.1 mol��L-1����������ϣ�д���кͷ�Ӧ�����ӷ���ʽ_____________��Ӧ����Һ������Ũ���ɴ�С����Ϊ_________________��
��4���ϳɰ��У���ȡ�����ķ���֮һ�ǣ�CO(g)+H2O(g)
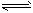 CO2(g)+H2(g) ��H<0�÷�Ӧ��427��Cʱ��ƽ�ⳣ����9�������Ӧ��ʼʱ��һ����̼��ˮ������Ũ�ȶ���0.1mol/L������һ����̼��ת���ʡ���д��������̣�
CO2(g)+H2(g) ��H<0�÷�Ӧ��427��Cʱ��ƽ�ⳣ����9�������Ӧ��ʼʱ��һ����̼��ˮ������Ũ�ȶ���0.1mol/L������һ����̼��ת���ʡ���д��������̣� ��1��4NH3(g)+5O2(g) 4NO(g)+6H2O(g) ��H= -905.8 kJ/mol
4NO(g)+6H2O(g) ��H= -905.8 kJ/mol
��2�����ԡ����ܣ���H<0���ǻ��Ҷ�����ķ�Ӧ
��3��NH3��H2O NH4++OH-��NH3��H2O+H+=NH4++H2O��c(Cl-)��c(NH4+)��C(H+) ��C(OH-)
NH4++OH-��NH3��H2O+H+=NH4++H2O��c(Cl-)��c(NH4+)��C(H+) ��C(OH-)
��4��25%
 4NO(g)+6H2O(g) ��H= -905.8 kJ/mol
4NO(g)+6H2O(g) ��H= -905.8 kJ/mol ��2�����ԡ����ܣ���H<0���ǻ��Ҷ�����ķ�Ӧ
��3��NH3��H2O
 NH4++OH-��NH3��H2O+H+=NH4++H2O��c(Cl-)��c(NH4+)��C(H+) ��C(OH-)
NH4++OH-��NH3��H2O+H+=NH4++H2O��c(Cl-)��c(NH4+)��C(H+) ��C(OH-) ��4��25%

��ϰ��ϵ�д�
�����Ŀ