��Ŀ����
�������д�����N2��O2��ֲ����������ȴҪ�Ӵ����ĵ��ʣ������죬����ˮ���ܼ�һ��������̬���ʣ������С��� ���ׯ�ڡ�֮˵���Իش�
(1)����������ˮ�ﺬ����̬���ʵı仯���̣��û�ѧ����ʽ��ʾ��______________________��
(2)ʹ�����еĵ���ת��Ϊ��̬���ʵĹ��̳�Ϊ___________��
(3)��������ˮ����CO2��pH=____������������(1)�ı仯���ټ���SO2���ܽ⣬pH���ͣ��γ����꣬��ɶԻ�������Ⱦ������ҪΣ����_______________��
(4)��д��SO2����ˮ�Ļ�ѧ����ʽ__________________��
(1)����������ˮ�ﺬ����̬���ʵı仯���̣��û�ѧ����ʽ��ʾ��______________________��
(2)ʹ�����еĵ���ת��Ϊ��̬���ʵĹ��̳�Ϊ___________��
(3)��������ˮ����CO2��pH=____������������(1)�ı仯���ټ���SO2���ܽ⣬pH���ͣ��γ����꣬��ɶԻ�������Ⱦ������ҪΣ����_______________��
(4)��д��SO2����ˮ�Ļ�ѧ����ʽ__________________��
(1)N2+O2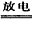 2NO��2NO+O2=2NO2��3NO2+H2O=2HNO3+NO
2NO��2NO+O2=2NO2��3NO2+H2O=2HNO3+NO
(2)���Ĺ̶�
(3)5.6���ƻ�ũ���ɭ�֡���ԭ��ʹ�����������ữ�����ḯʴ�������������ҵ�豸�����乤��ͨ�ŵ��µ�
(4)SO2+H2O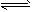 H2SO3
H2SO3
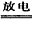 2NO��2NO+O2=2NO2��3NO2+H2O=2HNO3+NO
2NO��2NO+O2=2NO2��3NO2+H2O=2HNO3+NO (2)���Ĺ̶�
(3)5.6���ƻ�ũ���ɭ�֡���ԭ��ʹ�����������ữ�����ḯʴ�������������ҵ�豸�����乤��ͨ�ŵ��µ�
(4)SO2+H2O
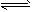 H2SO3
H2SO3 
��ϰ��ϵ�д�
�����Ŀ