��Ŀ����
��ⷨ�Ƽ����Ҫԭ���DZ���ʳ��ˮ�����ڴ���ˮ�к�����ɳ��Ca2+��Mg2+��Fe3+������1��ȡһ�����Ĵ��Σ������ձ��У�����������ˮ����ɴ���ˮ��
����2�������ˮ�м�������Լ���Ȼ����й��ˣ���ȥ�����������Һ�м������������ˮ��pH��
����3�����õ�����Һ����Ũ������ȴ���ᾧ�����ˡ���ɼ��þ��Ρ�
��ش��������⣺
(1)����ʵ���еĹ��˲�����Ҫ�ձ���____________��____________�Ȳ���������
(2)����2�г���Na2CO3��NaOH��BaCl2��Ϊ�����Լ������������Լ���˳��Ϊ_______________________��
(3)����2�У��жϼ���BaCl2�ѹ����ķ�����_______________________��
(4)����2�У��������������pH�ٹ��ˣ������ʵ��������Ӱ�죬��ԭ����_______
______________________________________________________��
(5)Ϊ���龫�δ��ȣ�������150 mL 0.2 mol��L-1 NaCl(����)��Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�е����������ǣ�

____________________________________________________________________��
____________________________________________________________________��
���ڶ���ʱ���ӣ���������Һ��Ũ��__________0.2 mol��L-1(����ڡ���С�ڡ�)�������ݲ��������̶��ߣ�Ӧ���õĴ���������________________________��
(1)������ ©��
(2)NaOH��BaCl2��Na2CO3��BaCl2��NaOH��Na2CO3
(3)ȡ������Һ���ϲ���Һ1��2���ڵζ����ϣ��ٵ���1��2��BaCl2��Һ������Һδ����ǣ������BaCl2�ѹ���(�����𰸺�������)
(4)�ڴ���������£����в��ֳ����ܽ⣬�Ӷ�Ӱ���Ƶþ��εĴ���
(5)δ�ò�����������δ����150 mL����ƿ С�� ��������
����������װ��������̨(����Ȧ)����������©�����ձ�����ֽ����ɡ����š����Ӳ����ӡ���ԭ��Ӧʹ���ʱ�Ϊ������������ᴿ���ʣ���ʹCa2+��Mg2+��Fe3+��![]() �����ӱ�Ϊ������Ca2+����̼�����ʽ������Mg2+����������þ������Fe3+��������������ʽ������
�����ӱ�Ϊ������Ca2+����̼�����ʽ������Mg2+����������þ������Fe3+��������������ʽ������![]() �������ᱵ��ʽ��������Ӧ�ȼ���BaCl2���ɡ��жϼ���BaCl2�ѹ����ķ�����Ӧ��ȡ����Һ�壬����BaCl2��̼���Ƽ��ɡ�����2�У��������������pH�ٹ��ˣ����ʹ���ֵ�����̼�������ܽ⣬��Ӧ���˺��ټ����ᡣ��ͼ�ɽ������(5)ȱ�ٲ���������������ƿ�ͺŲ��ԡ�
�������ᱵ��ʽ��������Ӧ�ȼ���BaCl2���ɡ��жϼ���BaCl2�ѹ����ķ�����Ӧ��ȡ����Һ�壬����BaCl2��̼���Ƽ��ɡ�����2�У��������������pH�ٹ��ˣ����ʹ���ֵ�����̼�������ܽ⣬��Ӧ���˺��ټ����ᡣ��ͼ�ɽ������(5)ȱ�ٲ���������������ƿ�ͺŲ��ԡ�

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
 ��ҵ�ϵ���Ƽ�ļ����������ӽ���Ĥ������Ҫԭ���DZ���ʳ��ˮ����ͼΪ���ӽ���Ĥ�����ԭ��ʾ��ͼ����ش��������⣺
��ҵ�ϵ���Ƽ�ļ����������ӽ���Ĥ������Ҫԭ���DZ���ʳ��ˮ����ͼΪ���ӽ���Ĥ�����ԭ��ʾ��ͼ����ش��������⣺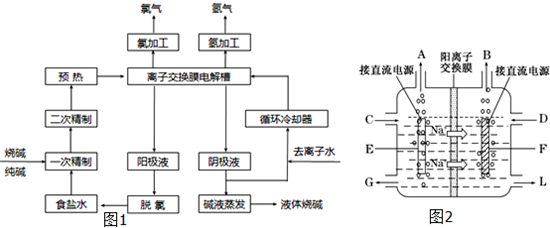
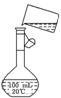 ��5��Ϊ���龫�δ��ȣ�������150 mL 0��2 mol/L NaCl�����Σ���Һ����ͼ�Ǹ�
��5��Ϊ���龫�δ��ȣ�������150 mL 0��2 mol/L NaCl�����Σ���Һ����ͼ�Ǹ�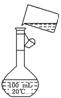 ��5��Ϊ���龫�δ��ȣ�������150 mL 0��2 mol/L NaCl�����Σ���Һ����ͼ�Ǹ�
��5��Ϊ���龫�δ��ȣ�������150 mL 0��2 mol/L NaCl�����Σ���Һ����ͼ�Ǹ�