��Ŀ����
(1)pH=2������һԪ��HX��HY����Һ�ֱ�ȡ50 mL������������þ�ۣ���ַ�Ӧ���ռ���H2������ֱ�ΪV(HX)��V(HY)����V(HX)��V(HY)����?�ٷ�Ӧ��ʼʱ��������H2������ ��?
�ڵ����ʵ���Ũ�ȵ�NaX��NaY��Һ�ļ��ԣ�NaX NaY(�������������=��)�������ǣ�?����������������?��?
(2)ij�о���ѧϰС���ͬѧ��ȡһ������������Na2SO3���壬��������600 ������ǿ�ȣ��ù���A�����ⶨǿ��ǰ������������ֲ��䡣Ϊ��һ��֤��Na2SO3������ǿ���������Ƿ�ֽ��Լ��������·ֽ��Ƿ���ȫ�����Ƕ�A��������ʵ�飺?

����ʵ�������Լ������غ㶨�ɺ�������ԭ��Ӧ���йع��ɣ������Ʋ����A�ijɷֿ�����Na2SO4��Na2SO3��Na2S2O3��Na2S��Na2O�е����֣���������������ּ��衣?
�Իش��������⣺?
����1����A��Һ���Ա�Na2SO3��Һ����ǿ�����������ɵ�Na2Sˮ�����£���Na2SO3����ֽ�Ļ�ѧ��Ӧ����ʽΪ������������������������?
��������C��H2S���ж�Na2SO3����ֽ��Ƿ���ȫ (��ǡ���)�����жϵ������ǣ�������������������������������?
����2����A��Һ���Ա�Na2SO3��Һ����ǿ�����������ɵ�Na2O����ˮ���£���Na2SO3����ֽ�Ļ�ѧ��Ӧ����ʽΪ��(δƽ��)?
����Na2SO3��������Na2S2O3+�� Na2SO4+�� Na2O������ƽ�û�ѧ��Ӧ����ʽ��?
��Ϊ��һ��ȷ�����ּ�����ȷ����С��ͬѧ�����й����ϵ�֪��Na2S2O3���ȶ��������ֽ⣬��������ж��������ּ�����ȷ ��?
(1)�����?
�ڣ�������HX��HY����Һ��������þ�۷�ӦHX������H2�࣬˵��c?(HX)��c(HY)��������c(H +)��ȣ���HX���Ա�HY������Խ�������ʵ���Ũ�ȵ�������Һ����Խǿ������NaX��NaY��Һ�ļ���NaX��NaY��
![]()
�ڷ�?
��ΪA��Һ������ϡ�����е���ɫ����(![]() + 2S2-+ 6H+ �� 3S��+ 3H2O)˵��Na2SO3û�зֽ���ȫ��??
+ 2S2-+ 6H+ �� 3S��+ 3H2O)˵��Na2SO3û�зֽ���ȫ��??
����2����4Na2SO3 ��1Na2S2O3 + 2Na2SO4 + 1Na2O???
�ڼ���1��ȷ��?
������(1)��������c(H+)�йأ����ڿ�ʼc(H+)=10-2mol��L-1����������Ҳ��ͬ����������HX��HY��Һ��������þ�۷�ӦHX������H2�࣬˵��c(HX)��c(HY)����c(H+)��ȣ���HX���Ա�HY������Խ����ͬŨ�ȵ�����ˮ��Խǿ������Խǿ������NaX��Һ�ļ��Դ���NaY��
(2)����������ԭ�������ɣ��н���������������Ԫ����+����-2����Ҳ�в�����Ԫ������+6�ۣ�����Na2SO4��![]() ?
?
��C��H2S���壬B�����ʣ�����һ����![]() ��S2-��H+����ʱ������S������Na2SO3δ��ȫ�ֽ⡣
��S2-��H+����ʱ������S������Na2SO3δ��ȫ�ֽ⡣
![]()
��Na2S2O3�����ֽ⣬���Բ�����Ϊ2��


 CH3COOH+OH-
CH3COOH+OH-
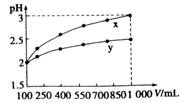
 ��2010?����һģ��ij��ѧѧϰС��Ϊ�о�HA��HB��MOH������Ե����ǿ�����������ʵ������ʵ�飺��pH�T2����������ҺHA��HB��pH=12��MOH����Һ��1mL���ֱ��ˮϡ�͵�1000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ���������������ݣ���ش��������⣺
��2010?����һģ��ij��ѧѧϰС��Ϊ�о�HA��HB��MOH������Ե����ǿ�����������ʵ������ʵ�飺��pH�T2����������ҺHA��HB��pH=12��MOH����Һ��1mL���ֱ��ˮϡ�͵�1000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ���������������ݣ���ش��������⣺