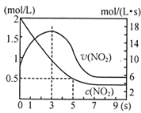
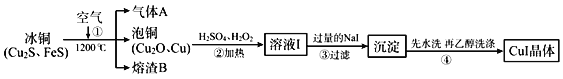
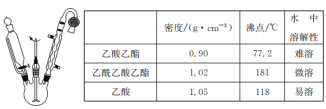
��Ŀ����
����Ŀ��������M(�ṹʽ����ͼ)����õĻ�ԭ������H��C�� Brown�� Schlesinger��1942����֥�Ӹ��ѧ���֣�����X��Y��Z��ԭ���������μ�С�IJ�ͬ����������Ԫ�أ�X��W��Z��W�����γ�ԭ�Ӹ�����Ϊ1:1��2:1�Ļ����Wԭ�ӵ������������ǵ��Ӳ�����3��������������ȷ���ǣ� ��
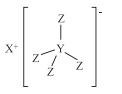
A.Y������������Ӧ��ˮ������һ��һԪ����
B.ZԪ��λ�ڵ�3���ڵڢ�A��
C.X��Z�γɵĻ������ˮ��Һ������
D.������M������Z2W2��Ӧ
���𰸡�A
��������
X��Y��Z��ԭ���������μ�С�IJ�ͬ����������Ԫ�أ�Xλ�ڵ������ڣ�Yλ�ڵڶ����ڣ�Zλ�ڵ�һ���ڣ���Z��H������Ϊ������M��XΪ+1�۵����ӣ���XΪNa��Y��H���ԭ����YH4-����Y��B����ΪWԭ�ӵ������������ǵ��Ӳ�����3������W��O��
�ɷ���֪��X��Na��Y��B��Z��H��W��O��
A��Y��B��B������������Ӧ��ˮ����H3BO3��H3BO3��һ��һԪ���ᣬA��ȷ��
B��Z��H��λ�ڵ�һ���ڵ���A�壬B����
C��X��Z�γɵĻ�������NaH��NaH+H2O= H2��+NaOH����X��Z�γɵĻ������ˮ��Һ�ʼ��ԣ�C����
D��������M��NaBH4��NaBH4����õĻ�ԭ����Z2W2��H2O2�dz��õ��������������ܷ���������ԭ��Ӧ��D����
��ѡD��