��Ŀ����
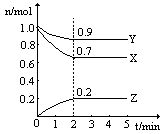
��.������㶨���ܱ������У�����2mol CO2��5mol H2��һ�������·�����Ӧ�� CO2(g) + 3H2(g)  CH3OH(g) + H2O(g) ��H =" -49.0" kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g) + H2O(g) ��H =" -49.0" kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��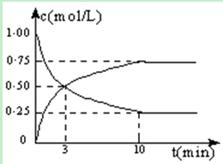
��1���ӷ�Ӧ��ʼ����10min��H2��ת����Ϊ ���� �������£���Ӧ��ƽ�ⳣ��K= �������ijһʱ�̱����¶Ȳ��䣬ֻ�ı�Ũ�ȣ�ʹc(CO2)=1.00mol/L��c(H2)=0.40mol/L��c(CH3OH)=c(H2O)=0.80mol/L����ƽ�� ��ѡ����ţ���
a���������ƶ� b���������ƶ�
c�����ƶ� d����ȷ��ƽ���ƶ�����
��2�����д�ʩ����ʹn(CH3OH)/n(CO2)������� ��ѡ����ţ���
a�������¶� b������He(g)��ʹ��ϵѹǿ����
c����H2O(g)����ϵ�з��� d���ٳ���l mol CH3OH(g)
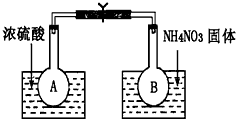
II������̼����ȼ�ϵ�أ�MCFS����������1889�ꡣ����һ��̼����ȼ�ϵ�أ���һ������Li2CO3��Na2CO3���ۻ����Ϊ����ʣ������¶�Ϊ650�棬�ڴ��¶�������Ϊ��������ú����CO��H2�������Ϊ1:1��ֱ����ȼ�ϣ��乤��ԭ����ͼ��ʾ����ش��������⣺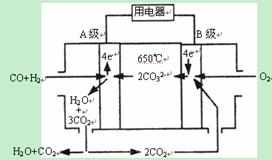
��1��A�缫�ĵ缫��Ӧ����ʽΪ ��
��2�������£���ʯī���缫���Դ˵�Դ���һ������CuSO4 ��Һ�����������������������ͬʱֹͣͨ�磬��������Һ�����Ϊ2L����Һ��pH=1��������ˮ�������H+������������������������ʵ����� ��
��.��1�� 90% �� K=" 144" �� a ��2�� c d
II����1�� CO+H2-4e-+2CO32-=3CO2+H2O ��2�� 0.1mol
���������������.��ͼ���ж�CO2��Ũ�Ƚ��ͣ�CH3OH(g)��Ũ�����ߣ� ���������Ϊ2L
CO2(g) + 3H2(g)  CH3OH(g) + H2O(g)
CH3OH(g) + H2O(g)
ʼ�� 2 5 0 0
ת���� 1.5 4.5 1.5 1.5
ƽ���� 0.5 0.5 1.5 1.5
H2��ת����Ϊ4.5��5=0.9
K=1.5/2��1.5/2�£�0.5/2 ��(0.5/2)3��=144
Qc=0.80��0.80�£�1.00��0.403��=10��144����ƽ�������ƶ���
��2��a�����¶�ƽ�������ƶ�����ֵ��С������b�����³���He(g)��ƽ�ⲻ�ƶ�����ֵ���䣬����c��������ƽ�������ƶ�����ֵ�����ȷ��d�ٳ���CH3OH(g)���京����ߣ���ֵ�����ȷ��
II����1��A�缫Ϊ������ע�����Ϊ̼���Σ� CO+H2-4e-+2CO32-=3CO2+H2O ��
��2�����CuSO4 ��Һ�����������������������Ȳ���ͭ���ʣ��������������ӦʽΪ
������4OH- + 4e- = O2��+ 2H2O
������Cu2+ + 2e- = Cu(��)
2H+ + 2e- = H2������
�����������������ȣ����ݵ����غ���ʽΪ4n-2n=0.1��2[����������H+ ��ȥ�������ĵ�H+ ������Һ��ʣ��H+ ] n=0.1mol
���㣺���黯ѧƽ�⼰�绯ѧ�й����⡣

��ˮú��ת���ɺϳ�����Ȼ��ϳɸ�����Ʒ��ʯ����Ʒ�ǻ����ļ�Ϊ��Ҫ������ȥˮ�������ˮú����Ҫ��H2��CO��CO2��������H2S��CH4��������ȥH2S�ɲ��ô���Ǵ�ת����������CH4ת����CO���õ�CO��CO2��H2�Ļ�����壬������ĺϳɼ״�ԭ������
(1)��ˮú������Ҫ��ѧ��Ӧ����ʽΪ��C(s)+H2O(g) CO(g)+H2(g)���˷�Ӧ�����ȷ�Ӧ��
CO(g)+H2(g)���˷�Ӧ�����ȷ�Ӧ��
�ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
������������̼��ת���ʵĴ�ʩ�� ��
| A������C(s) | B������H2O(g) | C�������¶� | D������ѹǿ |
 CO(g)+2H2O(g) ��H="-519" kJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ��(����������ͬ)
CO(g)+2H2O(g) ��H="-519" kJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ��(����������ͬ)��X��750��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Y��600��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Z��440��ʱ��Ч����ߣ���ʹ�淴Ӧ���ʼӿ�Լ1��106����
��֪����������Ϣ������Ϊ��������Ӧ��ѡ������˴����� (�X������Y����Z��)��ѡ��������� ��
�״�����Ϊ2l���͵�����ȼ�ϣ���ҵ��ͨ�����з�ӦI��II����CH4��H2OΪԭ�����Ʊ��״���
CH4(g)+H2O(g) 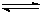 CO(g)+3H2 (g)����I CO(g)+2H2(g)
CO(g)+3H2 (g)����I CO(g)+2H2(g) 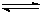 CH3OH(g) ����II��
CH3OH(g) ����II��
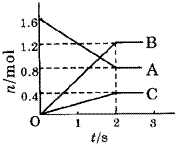
��1����1.0 mol CH4��2.0 mol H2O(g)ͨ���ݻ�Ϊ100L��Ӧ�ң���һ�������·�����ӦI��CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��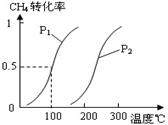
����֪100��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����Ϊ____________________��
��ͼ�е�P1_________P2���<������>����=������100��ʱƽ�ⳣ����ֵΪ__________ ��
��2����ѹǿΪ0.1 MPa�����£� ��a mol CO�� 3a mol H2�Ļ�������ڴ��������£��Է���Ӧ�����ɼ״���
�۸÷�Ӧ�ġ�H ____ 0���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ���________��
| A�������¶� | B����CH3OH(g)����ϵ�з��� |
| C������He��ʹ��ϵ��ѹǿ���� | D���ٳ���1mol CO��3mol H2 |
| ʵ���� | T(��) | N(CO)/n(H2) | P��Mpa�� |
| i | 150 | 1/3 | 0.1 |
| ii | | | 5 |
| iii | 350 | | 5 |
a�������ϱ��ո�������ʣ���ʵ���������ݡ�
b�����ݷ�ӦII���ص㣬�ڸ���������ͼ�У�������5MPa������CO��ת�������¶ȱ仯����������ʾ��ͼ��������ѹǿ��
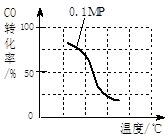
�ϳɰ������Ĵ����������˹��̵�����Ҫ;�������о�������ȷ������ָ�����ϳɰ���Ӧ��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ���£�
| �� �ȣ��棩 | 360 | 440 | 520 |
| Kֵ | 0.036 | 0.010 | 0.0038 |
��1����д����ҵ�ϳɰ��Ļ�ѧ����ʽ_________________________________________��
�����ϱ����ݿ�֪�÷�ӦΪ���ȷ�Ӧ��������_____________________________________��
�������ϣ�Ϊ������ƽ��ʱH2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ�ǡ�������ţ�
a������ѹǿ b��ʹ�ú��ʵĴ���
c�������¶� d����ʱ����������е�NH3
��2��ԭ����H2��ͨ����Ӧ CH4(g) + H2O (g)
 CO(g) + 3H2(g) ��ȡ����֪�÷�Ӧ�У�����ʼ������е�
CO(g) + 3H2(g) ��ȡ����֪�÷�Ӧ�У�����ʼ������е�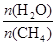 �㶨ʱ���¶ȡ�ѹǿ��ƽ������CH4������Ӱ������ͼ��ʾ��
�㶨ʱ���¶ȡ�ѹǿ��ƽ������CH4������Ӱ������ͼ��ʾ��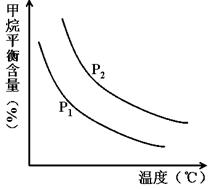
��ͼ�У��������߱�ʾѹǿ�Ĺ�ϵ�ǣ�P1________P2�����������������������
�ڸ÷�ӦΪ_____________��Ӧ������ȡ����ȡ�����
��3��ԭ����H2����ͨ����ӦCO(g) + H2O(g)
 CO2 (g) + H2(g) ��ȡ��
CO2 (g) + H2(g) ��ȡ����T ��ʱ�����ݻ��̶�Ϊ5 L�������г���1 molˮ������1 mol CO����Ӧ��ƽ����CO��Ũ��Ϊ0.08 mol��L-1����ƽ��ʱCO��ת����Ϊ______�����¶��·�Ӧ��ƽ�ⳣ��KֵΪ_________��
�ڱ����¶���ΪT �棬�ı�ˮ������CO�ij�ʼ���ʵ���֮�ȣ������������з�Ӧ�����������ܹ�˵����ϵ����ƽ��״̬����_____________������ţ���
a��������ѹǿ����ʱ��ı�
b�����������ܶȲ���ʱ��ı�
c����λʱ��������a mol CO2��ͬʱ����a mol H2
d���������n(CO) : n(H2O) : n(CO2) : n(H2) �� 1: 16 : 6 : 6
��. ��ˮ��Һ�гȺ�ɫ��Cr2O72-���ɫ��CrO42-������ƽ���ϵ��
Cr2O72-��H2O  2CrO42-��2H+����K2Cr2O7����ˮ���ϡ��Һ�dz�ɫ��
2CrO42-��2H+����K2Cr2O7����ˮ���ϡ��Һ�dz�ɫ��
��1����������Һ�м���NaOH��Һ����Һ�� ɫ����Ϊ ��
��2�����Ѽ���NaOH��Һ�ģ�1�����ټ������ϡH2SO4������Һ�� ɫ����Ϊ ��
��3����ԭ��Һ�м���Ba(NO3)2��Һ����֪BaCrO4Ϊ��ɫ��������ƽ���� �����ƶ�����Һ��ɫ�� ��������������dz�����䡱��
��.ʵ������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺
1.����100mL 0.10mol/L NaOH����Һ��
2.ȡ20.00mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�NaOH��Һ���еζ���
3.�ظ������ζ�����2��3�Σ���¼�������¡�
| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� | �ζ����ʱ��NaOH��Һ����������mL�� | ����������Һ����� ��mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.58 | 20.00 |
| 3 | 0.10 | 22.60 | 20.00 |
��1���ζ��ﵽ�յ�������� ����ʱ��ƿ����Һ��pH��ΧΪ ��
��2�������������ݣ��ɼ�����������Ũ��ԼΪ ��
��3����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ �IJ�����Ȼ��ѹ������ʹ���첿�ֳ�����Һ��



�� �� ��
��4��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ��� ����ѡ�۷֣���
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ��
E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶���
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
 H2+I2
H2+I2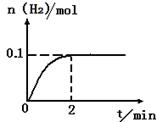
 2SO3(g) + Q�����˷�Ӧ��ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����_________ (�����)
2SO3(g) + Q�����˷�Ӧ��ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����_________ (�����)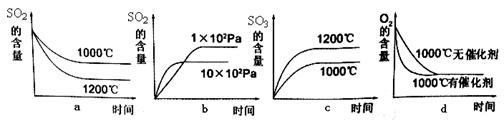
 N2O4(g) ��H= -52.7kJ��mol-1��ij����С��Ϊ��̽���¶Ⱥ�ѹǿ�Ի�ѧƽ���Ӱ�죬������������ʵ�飺
N2O4(g) ��H= -52.7kJ��mol-1��ij����С��Ϊ��̽���¶Ⱥ�ѹǿ�Ի�ѧƽ���Ӱ�죬������������ʵ�飺