��Ŀ����
ʵ��������240ml 0.2mol?L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡNa2CO3?10H2O����______ g��
��2������ʱӦ��ѡ��������ƽ��ҩ�ס��ձ�������������ͷ�ι��⣬����Ҫ�õ���������______��
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е�______��������ţ�
��4������ʱ��һ��ɷ�Ϊ���¼������裺�ٳ������ڼ��㡡���ܽ⡡��ҡ�ȡ���ת�� ��ϴ�� �߶��� ����ȴ������ȷ�IJ���˳��Ϊ______��
��5����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵���______������ѡ��
A�����ǽ�ϴ��Һת������ƿ��
B������ƿϴ�Ӻ��ڱ���ˮ���δ�����ﴦ����
C�����ݡ�ҡ�ȡ����ú��ְ�����ڿ̶����ּ�ˮ���̶��ߣ�
D������ʱ���ӿ̶��ߣ�
E��Na2CO3?10H2O��ʧȥ���ֽᾧˮ��
�⣺��1����������Һ�����Ϊ240ml��������ƿ�Ĺ��û��240ml��ֻ��ѡ��250ml��Na2CO3�����ʵ���n=cV=0.25L��0.2mol?L-1=0.05mol��Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO3?10H2O������0.05mol��286g/mol=14.3g���ʴ�Ϊ��14.3��
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ��
��3��������ƿ�ϱ����¶ȡ��������̶��ߣ���ѡ���٢ۢݣ�
��4�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��5��A�����ǽ�ϴ��Һת������ƿ�����ʵ�����ƫС��Ũ��ƫС����A��ȷ��
B������ƿϴ�Ӻ��ڱ���ˮ���δ�����ﴦ������Һ��������䣬Ũ�Ȳ��䣬��B����
C�����ݡ�ҡ�ȡ����ú��ְ�����ڿ̶����ּ�ˮ���̶��ߣ���Һ�����ƫ��Ũ��ƫС����C��ȷ��
D������ʱ���ӿ̶��ߣ���Һ�����ƫС��Ũ��ƫ��C����
E��Na2CO3?10H2O��ʧȥ���ֽᾧˮ�����ʵ�����ƫ��Ũ��ƫ��E����
�ʴ�Ϊ��A��C��
��������1������n=cv��������Na2CO3�����ʵ���������Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3?10H2O��������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ��������
��3����������ƿ�ϱ����¶ȡ��������̶��ߣ�
��4������ʵ������IJ��裻
��5������c=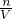 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
���������⿼����һ�����ʵ���Ũ����Һ�������Լ����������ѶȲ���ע��ʵ��Ļ�������������ע�����
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ��
��3��������ƿ�ϱ����¶ȡ��������̶��ߣ���ѡ���٢ۢݣ�
��4�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��5��A�����ǽ�ϴ��Һת������ƿ�����ʵ�����ƫС��Ũ��ƫС����A��ȷ��
B������ƿϴ�Ӻ��ڱ���ˮ���δ�����ﴦ������Һ��������䣬Ũ�Ȳ��䣬��B����
C�����ݡ�ҡ�ȡ����ú��ְ�����ڿ̶����ּ�ˮ���̶��ߣ���Һ�����ƫ��Ũ��ƫС����C��ȷ��
D������ʱ���ӿ̶��ߣ���Һ�����ƫС��Ũ��ƫ��C����
E��Na2CO3?10H2O��ʧȥ���ֽᾧˮ�����ʵ�����ƫ��Ũ��ƫ��E����
�ʴ�Ϊ��A��C��
��������1������n=cv��������Na2CO3�����ʵ���������Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3?10H2O��������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ��������
��3����������ƿ�ϱ����¶ȡ��������̶��ߣ�
��4������ʵ������IJ��裻
��5������c=
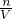 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ����������⿼����һ�����ʵ���Ũ����Һ�������Լ����������ѶȲ���ע��ʵ��Ļ�������������ע�����

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ
(10��)ʵ��������240ml 0.2mol��L-1��Na2CO3��Һ���ش������� �⣺
�⣺
��1��Ӧ��������ƽ��ȡNa2CO3��10H2O���� g��
��2������ʱӦ��ѡ��������ƽ��ҩ�ס��ձ�������������ͷ�ι��⣬����Ҫ�õ��������� ��
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ��������ţ�
��4������ʱ ��һ��ɷ�Ϊ���¼������裺�ٳ��� �ڼ��� ���ܽ�
��һ��ɷ�Ϊ���¼������裺�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�Ƣ�ϴ�Ӣ߶��ݢ���ȴ������ȷ�IJ���˳��Ϊ ��
��ҡ�� ��ת�Ƣ�ϴ�Ӣ߶��ݢ���ȴ������ȷ�IJ���˳��Ϊ ��
��5����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��(��ѡ)
| A�����ǽ�ϴ��Һת������ƿ�� |
| B������ƿϴ�Ӻ��ڱ���ˮ���δ�����ﴦ���� |
| C�����ݡ�ҡ�ȡ����ú��ְ�����ڿ̶����ּ�ˮ���̶��ߣ� |
| D������ʱ���ӿ̶��ߣ� |