��Ŀ����
����Ŀ��������������(Na2S2O4)�ֳƱ��շۣ���ӡˢ��ҵ����Ҫ�Ļ�ԭ����ij����С���������ʵ�飺
��.��������
��������������(Na2S2O4)��һ�ְ�ɫ��ĩ��������ˮ���������Ҵ���
��2Na2S2O4��4HCl===4NaCl��S����3SO2����2H2O��
Na2S2O3��2HCl===2NaCl��S����SO2����H2O��
��.�Ʊ�����
75 ��ʱ�������ƺʹ�������Ҵ���Һ�У�ͨ��SO2���з�Ӧ������䷴Ӧ�Ļ�ѧ����ʽ��
________HCOONa��________Na2CO3��________===________Na2S2O4��________CO2��______
��ȴ��40��50 �棬���ˣ���________ϴ�ӣ������Ƶ�Na2S2O4��
��.Na2S2O4������
(1)Na2S2O4��Һ�ڿ������ױ�����������С��ⶨ0.050 mol��L��1 Na2S2O4��Һ�ڿ�����pH�仯��ͼ��ʾ��
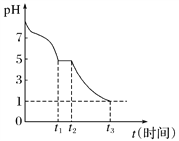
0��t1����Ҫ����HSO![]() ������pH�仯ͼ��HSO
������pH�仯ͼ��HSO![]() �ĵ���̶�________(���������)ˮ��̶ȡ�
�ĵ���̶�________(���������)ˮ��̶ȡ�
0��t1�η�����Ӧ�����ӷ���ʽΪ_____________��
t3ʱ��Һ�д��ڵ���Ҫ�����ӵķ�����_________________��
(2)������������Na2S2O4������ȫ�ֽ⣻�õ��������Na2SO3��Na2S2O3��________(�ѧʽ)���塣
�������ʵ����֤������Na2S2O3���ڣ�����±������ݡ�
(��ѡ����Լ���ϡ���ᡢϡ���ᡢBaCl2��Һ��KMnO4��Һ)
ʵ�鲽��(��Ҫ��д�������������) | Ԥ�ڵ�ʵ������ͽ��� |
______ | _________ |
���𰸡� 2 1 4 SO2 2 3 1 H2O �Ҵ� �� 2S2O42-��O2��2H2O===4HSO3- SO42- SO2 ȡ������ȫ�ֽ��Ĺ���������Թ��У�����ϡ���� ���е���ɫ��������������Na2S2O3����
��������II����Ӧ��SԪ�صĻ��ϼ۴�+4�۽��͵�+3�ۣ��õ�1�����ӣ���������̼Ԫ�صĻ��ϼ۴�+2�����ߵ�+4�ۣ�����ݵ��ӵ�ʧ�غ��ԭ���غ��֪��Ӧ�Ļ�ѧ����ʽΪ2HCOONa+Na2CO3+4SO2=2Na2S2O4+3CO2+H2O����������������һ�ְ�ɫ��ĩ��������ˮ���������Ҵ�����˿������Ҵ�ϴ�Ӳ�Ʒ���ʴ�Ϊ��2�� 1��4��SO2��2 ��3��1 H2O���Ҵ���
III��(1)0��t1����Ҫ����HSO![]() ������pH�仯ͼ����Һ�����ԣ���HSO
������pH�仯ͼ����Һ�����ԣ���HSO![]() �ĵ���̶���ˮ��̶���Na2S2O4��Һ�ڿ������ױ������������������ƣ���0��t1�η������ӷ�Ӧ����ʽΪ 2S2O42-+O2+2H2O=4HSO3-��t3ʱ��Һ��pH=1��˵����Һ�����Խ�ǿ��������������Ʊ�����Ϊ�������ƣ�����Һ����Ҫ�����ӷ�����SO42-���ʴ�Ϊ������2S2O42-+O2+2H2O=4HSO3-��SO42-��
�ĵ���̶���ˮ��̶���Na2S2O4��Һ�ڿ������ױ������������������ƣ���0��t1�η������ӷ�Ӧ����ʽΪ 2S2O42-+O2+2H2O=4HSO3-��t3ʱ��Һ��pH=1��˵����Һ�����Խ�ǿ��������������Ʊ�����Ϊ�������ƣ�����Һ����Ҫ�����ӷ�����SO42-���ʴ�Ϊ������2S2O42-+O2+2H2O=4HSO3-��SO42-��
(2)������������Na2S2O4������ȫ�ֽ⣬�õ��������Na2SO3��Na2S2O3�����壬�����������������������Na2S2O3��S��+2�ۣ������������ԭ��Ӧ�е��ӵ�ʧ�غ��֪����Ӧ����SO2�����ݷ�ӦNa2S2O3+2HCl=2NaCl+S��+SO2��+H2O��֪Ҫ������������ƣ���ֻ��Ҫȡ������ȫ�ֽ�Ĺ���������Թ��У�����ϡ���ᣬ����е���ɫ����������Na2S2O3���ڣ��ʴ�Ϊ��SO2��ȡ������ȫ�ֽ��Ĺ���������Թ��У�����ϡ���� �����е���ɫ��������������Na2S2O3������

 ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�����Ŀ����ɫ��Һ�к��У���Na������Ba2������Cl������Br������SO![]() ����SO
����SO![]() ����Fe2�������е�һ�ֻ��֣����ν�������ʵ�飬��ÿ�������Լ����������۲쵽���������£�
����Fe2�������е�һ�ֻ��֣����ν�������ʵ�飬��ÿ�������Լ����������۲쵽���������£�
���� | ���� | ���� |
�� | ��pH��ֽ���� | ��Һ��pH����7 |
�� | ����Һ�еμ���ˮ���ټ���CCl4������ | CCl4��ʳȺ�ɫ |
�� | ȡ�ڵ��ϲ���Һ������Ba��NO3��2��Һ��ϡHNO3 | �а�ɫ�������� |
�� | ���۹��ˣ�����Һ�м���AgNO3��Һ��ϡHNO3 | �а�ɫ�������� |
��������ʵ�������ж����½�������ȷ���ǣ�������
A. �϶����е������Ǣ٢ܢ� B. �϶�û�е������Ǣڢݢ�
C. ���ܺ��е������Ǣ٢ڢ� D. ����ȷ���������Ǣ٢ۢ�



