��Ŀ����
11�����г��������¼ס��ҡ���������Һ����Ϊ0.1mol/L��NaOH��Һ����Ϊ0.1mol/L��HCl��Һ����Ϊ 0.1mol/L ��CH3COOH��Һ���Իش��������⣺��1������Һ��pH=13��
��2������Һ�д��ڵĵ���ƽ��ΪCH3COOH?CH3COO-+H+��H2O?OH-+H+ ���õ��뷽��ʽ��ʾ����
��3���ס��ҡ���������Һ����ˮ�������c��OH-���Ĵ�С��ϵΪ������=�ң�
��4��ijͬѧ�ü���Һ�ֱ�ζ�20.00mL����Һ��20.00mL����Һ���õ���ͼ��ʾ�����ζ����ߣ�������й����⣺
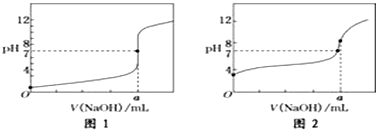
�ټ���Һ�ζ�����Һ��������ͼ2���ͼ1����ͼ2������
��a=20.00 mL��
���� ��1��0.1mol•L-1��NaOH��Һ��c��OH-��=0.1mol/L������Kw=c��H+��•c��OH-��������Һ��c��H+�����ٸ���pH=-lgc��H+��������Һ��pHֵ��
��2����Һ�д��ڵ���ƽ�⣬Ӧ����������ʣ�
��3����������ˮ���룬�����������ӵ��δٽ�ˮ���룻
��4��������Ϊ������ʣ��ζ��յ�ʱ����Һ�ʼ��ԣ�
��NaOH������ǡ�÷�Ӧʱ������20mlNaOH��Һ����������ǿ���Σ���Һ�ʼ��ԣ�
��� �⣺��1��0.1mol•L-1��NaOH��Һ��c��OH-��=0.1mol/L������Һ��c��H+��=$\frac{1��1{0}^{-14}}{0.1}$mol/L=10-13mol/L���ʸ���Һ��pH=-lg10-13=13���ʴ�Ϊ��13��
��2��0.1mol•L-1��CH3COOH��Һ�д����ܼ�ˮ��ˮΪ������ʣ���������Ϊ������ʣ����ڵ���ƽ��ΪCH3COOH?CH3COO-+H+��H2O?OH-+H+��
�ʴ�Ϊ��CH3COOH?CH3COO-+H+��H2O?OH-+H+��
��3����������ˮ���룬�����������ӵ��δٽ�ˮ���룬������������ʣ��������ơ��Ȼ�����ǿ����ʣ�������ͬ���ʵ���Ũ�ȵ������������������ƣ�������ˮ�����������������Ũ��С�ڴ��ᣬ��ͬ���ʵ���Ũ�ȵ�������������ƶ�ˮ�������Ƴ̶���ȣ�������ˮ�����������������Ũ�ȵ�������������Һ��ˮ�ĵ��룬����ˮ���������������Ũ�ȴ�С˳���ǣ�������=�ң�
�ʴ�Ϊ��������=�ң�
��4���ٴ���Ϊ������ʣ��ζ�������pH�仯�����Ỻ�����ζ��յ�ʱ��Һ�ʼ��ԣ���ζ�������Һ��������ͼ2���ʴ�Ϊ��ͼ2��
�ڵζ��յ�ʱn��CH3COOH��=n��NaOH������a=20.00mL����Ӧ�����˴����ƣ���������ӷ���ˮ�⣬CH3COO-+H2O?CH3COOH+OH-����Һ��ʾ����pH��7��
�ʴ�Ϊ��20.00��
���� �����ۺϿ�������ϵĶ����жϼ���ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ��Һ���������ҺpH�Ĺ�ϵ���ͼ������ж��ǽ����ؼ�������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �������� | �������� | ʵ��Ԥ�� | |
| A | Ũ��ˮ | ��̪��Һ | ������Һ��Ϊ��ɫ |
| B | Ũ���� | ����KI��Һ | ������Һ��Ϊ��ɫ |
| C | Ũ���� | Ũ��ˮ | ���ձ����а��� |
| D | ���͵���������Һ | ϡ��ˮ | ���������Ա仯 |
| A�� | A | B�� | B | C�� | C | D�� | D |
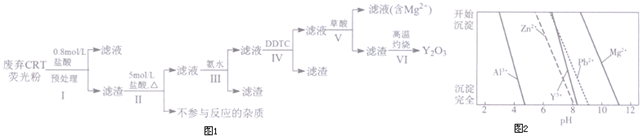
��֪���ٷ���CRTӫ��۵Ļ�ѧ��ɣ�ijЩ�����뷴Ӧ������δ�г������һ��ʾ��
�ڲ�ͬ���ӳ�����pH��ͼ2��ʾ��
| �ɷ� ����/% �� | Y2O3 | ZnO | Al2O3 | PbO2 | MgO |
| Ԥ����ǰ | 24.28 | 41.82 | 7.81 | 1.67 | 0.19 |
| Ԥ������ | 68.51 | 5.42 | 4.33 | 5.43 | 0.50 |
��2����������л���ɫ����������÷�Ӧ�Ļ�ѧ����ʽΪPbO2+4HCl$\frac{\underline{\;\;��\;\;}}{\;}$PbCl2+Cl2��+2H2O��
��3��������з�������Ҫ��Ӧ�����ӷ���ʽΪAl3++3NH3•H2O=Al��OH��3��+3NH4+��
��4��������г����Լ�DDTC��ȥ������������Zn2+��Pb2+���䲻��ͨ��ֱ�ӼӼ�ķ�����ȥ��ԭ��ΪZn2+��Pb2+��Y3+������pH�����������ͬʱ�����������룮
��5������V��Y3+������ȫʱ���豣֤�μӲ�������Һ��c��C2O42-��������2.0��10-6mol/L��
����֪��������Ũ��С��10-5mol/Lʱ�������ʹ���ȫ��K��[Y2��C2O4��3]=8.0��10-28
��6��������в����Ƹ����������ȿ��Եõ�Y2O3���÷�Ӧ�Ļ�ѧ����ʽΪY2��C2O4��3$\frac{\underline{\;\;��\;\;}}{\;}$Y2O3+3CO��+3CO2����
������ԭ��ص������ǣ�Cu���缫��Ӧʽ��2H++2e-=H2����
�������������Һ��Ϊ����ͭ��Һ�������ǣ�Zn�������缫��Ӧʽ��Zn-2e-=Zn2+�������缫��Ӧʽ��Cu2++2e-�TCu������������ͬ�ĵ���ʱ�����������������Ϊ12.9g��
��2����A��B��C��D���ֽ������±���װ�ý���ʵ�飮
| װ�� |  |  |  |
| ���� | ���۽���A�����ܽ� | C���������� | A����������� |
��װ�ü��и����ĵ缫��Ӧʽ�ǣ�A-2e-�TA2+��
��װ�����������ĵ缫��Ӧʽ�ǣ�Cu2++2e-�TCu��
��װ�ñ�����Һ��pH�����������С�����䡱����
�����ֽ��������ǿ������˳����D��A��B��C��
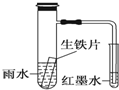
| A�� | ����Ƭ�е�̼��ԭ��صĸ�����������ԭ��Ӧ | |
| B�� | ��ˮ���Խ�ǿ������Ƭʼ�շ������ⸯʴ | |
| C�� | ��֧�Թ�����Һ������ǿ | |
| D�� | īˮ����ʱ��̼�缫��ӦʽΪO2+2H2O+4e-�T4OH- |
| A�� | $\frac{c��O{H}^{-}��•c��N{H}_{4}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$ | B�� | $\frac{c��N{H}_{3}•{H}_{2}O��}{c��O{H}^{-}��}$ | ||
| C�� | c��H+����c��OH-���ij˻� | D�� | OH-�����ʵ��� |
| A�� | 3d | B�� | 5f | C�� | 6p | D�� | 7s |
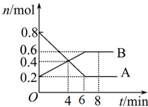 ij�¶�ʱ����2L�ܱ�������ijһ��Ӧ��A��B���ʵ�����ʱ��仯��������ͼ��ʾ��
ij�¶�ʱ����2L�ܱ�������ijһ��Ӧ��A��B���ʵ�����ʱ��仯��������ͼ��ʾ�� ��
�� ��
�� ��
��