��Ŀ����
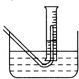 Na2O2��ˮ�ķ�Ӧʵ����Na2O2+2H2O�T2NaOH+H2O2����Ӧ���ȣ���Ӧ�ų�������ʹ����H2O2���ȷֽ⣺2H2O2�T2H2O+O2����Ϊ�˲ⶨij�������ƹ���Ĵ��ȣ���������ʵ�飺�ٳ�ȡ�������ƹ���2.00g���ڰ���Щ�������ƹ���������������õ����巢��װ���У�������������еμ�ˮ����ijһ��Ͳ��ˮ��������Ͳ��Һ����112mL��ǡ����ˮ����Һ����ƽ���ܽ���ƿ�е�Һ��ת�Ƶ�250mL������ƿ�У�ϴ�Ӳ���ϴ��ҺҲת������ƿ��Ȼ���������ˮ�����ݣ�ʹҺ��ǡ����̶����У�������Һ����ȡ25.00mL����ƿ�е�Һ�壬������ƿ�У��ù�����ϡ�����ữ��Ȼ����0.01mol/L��KMnO4��Һȥ�ζ������յ�ʱ��ȥ��24.20mLKMnO4��Һ����ʱ��ȫ����Mn2+���ڣ�
Na2O2��ˮ�ķ�Ӧʵ����Na2O2+2H2O�T2NaOH+H2O2����Ӧ���ȣ���Ӧ�ų�������ʹ����H2O2���ȷֽ⣺2H2O2�T2H2O+O2����Ϊ�˲ⶨij�������ƹ���Ĵ��ȣ���������ʵ�飺�ٳ�ȡ�������ƹ���2.00g���ڰ���Щ�������ƹ���������������õ����巢��װ���У�������������еμ�ˮ����ijһ��Ͳ��ˮ��������Ͳ��Һ����112mL��ǡ����ˮ����Һ����ƽ���ܽ���ƿ�е�Һ��ת�Ƶ�250mL������ƿ�У�ϴ�Ӳ���ϴ��ҺҲת������ƿ��Ȼ���������ˮ�����ݣ�ʹҺ��ǡ����̶����У�������Һ����ȡ25.00mL����ƿ�е�Һ�壬������ƿ�У��ù�����ϡ�����ữ��Ȼ����0.01mol/L��KMnO4��Һȥ�ζ������յ�ʱ��ȥ��24.20mLKMnO4��Һ����ʱ��ȫ����Mn2+���ڣ�
��1����500ml��250ml��150ml����Ͳ���ã�Ӧѡ������Ϊ______����Ͳ
��2����ʵ��Ӧѡ��______�����ʽ����ʽ�����ζ���
��3���ڲ���۲����������ʱ���������ƿ����Ͳ�ڵ����嶼��ȴ������ʱ���У���ʱ��Ͳ�ڵ�Һ�����ˮ����Һ�棨��ͼ��������������ʹNa2O2�Ĵ���______���ƫ�ߡ���ƫ�͡����䡱����Ӧ���еIJ�����______
��4���ڲ�������õ���������������ƿ���Ҫ______
��5���ڲ�����з�Ӧ�����ӷ���ʽ��______�ж��ζ��ﵽ�յ��������______
��6���ù������ƵĴ���Ϊ______���ðٷ�����ʾ������һλС����ʵ���еõ����������������Ϊ��״���£�
�⣺��1������ͲʱӦѡ������ӽ�����Ͳ���Լ����������ռ�112mL����Ӧѡ��150mL����Ͳ���ʴ�Ϊ��150ml��
��2�����������ǿ�����Ե����ʣ�Ӧѡ����ʽ�ζ��ܣ��ʴ�Ϊ����ʽ��
��3���ڲ���۲����������ʱ���������ƿ����Ͳ�ڵ����嶼��ȴ������ʱ���У���ʱ��Ͳ�ڵ�Һ�����ˮ����Һ�棬����������ʹ�ռ������������ƫ�ߣ����ƫ�ߣ�Ӧ��������Ͳ�����ƣ�ʹ��Ͳ��Һ��ǡ����ˮ����Һ����ƽ���ʴ�Ϊ��ƫ�ߣ�Ӧ��������Ͳ�����ƣ�ʹ��Ͳ��Һ��ǡ����ˮ����Һ����ƽ��
��4������һ�����ʵ���Ũ�ȵ���Һ��Ҫ�IJ�������������������ͷ�ιܣ��Լ�һ����������ƿ���ʴ�Ϊ������������ͷ�ιܣ�
��5��������ؾ��������ԣ�˫��ˮ���л�ԭ�ԣ����߿��Է�����������Ӧ����2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O��������ؾ�����ɫ������Ҫָʾ�����жϵζ��յ㣬����Һ�պó���dz�Ϻ�ɫ�����ڰ�����ڲ���ɫ�������ﵽ�ζ��յ㣬�ʴ�Ϊ��2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O����Һ�պó���dz�Ϻ�ɫ�����ڰ�����ڲ���ɫ��
��6���⣺˫��ˮ�ֽ����������2H2O2�T2H2O+O2����������112mL����ʱ���ֽ��˫��ˮ =0.01��mol�������ݷ�Ӧ2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O���ʣ��˫��ˮ����
=0.01��mol�������ݷ�Ӧ2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O���ʣ��˫��ˮ���� ��0.006mol������˫��ˮ������=0.01mol+0.006mol=0.016mol�����ݷ�ӦNa2O2+2H2O�T2NaOH+H2O2����֪�������Ƶ���Ϊ0.0016mol������=
��0.006mol������˫��ˮ������=0.01mol+0.006mol=0.016mol�����ݷ�ӦNa2O2+2H2O�T2NaOH+H2O2����֪�������Ƶ���Ϊ0.0016mol������= =62.4%���ʴ�Ϊ��62.4%��
=62.4%���ʴ�Ϊ��62.4%��
��������1������Ͳ��ȡҺ��ʱӦѡ������ӽ�����Ͳ���Լ�����
��2�����Ժ�ǿ�����Ե�����Ӧѡ����ʽ�ζ��ܣ�
��3����ˮ�����������ʱ����Ͳ�ڵ�Һ��Ҫ��ˮ����Һ����ƽ�����ý���dz��³�ѹ�µ����������
��4����������һ�����ʵ���Ũ�ȵ���Һ��Ҫ���������ش�
��5��������ؾ��������ԣ�˫��ˮ���л�ԭ�ԣ����߿��Է�����������Ӧ��������ؾ�����ɫ��
��6���������ƵĴ���= ��100%��
��100%��
������������һ�����������⣬����ѧ�������ͽ��������������ۺ��Խ�ǿ���ѶȺܴ�
��2�����������ǿ�����Ե����ʣ�Ӧѡ����ʽ�ζ��ܣ��ʴ�Ϊ����ʽ��
��3���ڲ���۲����������ʱ���������ƿ����Ͳ�ڵ����嶼��ȴ������ʱ���У���ʱ��Ͳ�ڵ�Һ�����ˮ����Һ�棬����������ʹ�ռ������������ƫ�ߣ����ƫ�ߣ�Ӧ��������Ͳ�����ƣ�ʹ��Ͳ��Һ��ǡ����ˮ����Һ����ƽ���ʴ�Ϊ��ƫ�ߣ�Ӧ��������Ͳ�����ƣ�ʹ��Ͳ��Һ��ǡ����ˮ����Һ����ƽ��
��4������һ�����ʵ���Ũ�ȵ���Һ��Ҫ�IJ�������������������ͷ�ιܣ��Լ�һ����������ƿ���ʴ�Ϊ������������ͷ�ιܣ�
��5��������ؾ��������ԣ�˫��ˮ���л�ԭ�ԣ����߿��Է�����������Ӧ����2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O��������ؾ�����ɫ������Ҫָʾ�����жϵζ��յ㣬����Һ�պó���dz�Ϻ�ɫ�����ڰ�����ڲ���ɫ�������ﵽ�ζ��յ㣬�ʴ�Ϊ��2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O����Һ�պó���dz�Ϻ�ɫ�����ڰ�����ڲ���ɫ��
��6���⣺˫��ˮ�ֽ����������2H2O2�T2H2O+O2����������112mL����ʱ���ֽ��˫��ˮ
 =0.01��mol�������ݷ�Ӧ2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O���ʣ��˫��ˮ����
=0.01��mol�������ݷ�Ӧ2MnO4-+5H2O2+6H+=2Mn2++5O2��+8H2O���ʣ��˫��ˮ���� ��0.006mol������˫��ˮ������=0.01mol+0.006mol=0.016mol�����ݷ�ӦNa2O2+2H2O�T2NaOH+H2O2����֪�������Ƶ���Ϊ0.0016mol������=
��0.006mol������˫��ˮ������=0.01mol+0.006mol=0.016mol�����ݷ�ӦNa2O2+2H2O�T2NaOH+H2O2����֪�������Ƶ���Ϊ0.0016mol������= =62.4%���ʴ�Ϊ��62.4%��
=62.4%���ʴ�Ϊ��62.4%����������1������Ͳ��ȡҺ��ʱӦѡ������ӽ�����Ͳ���Լ�����
��2�����Ժ�ǿ�����Ե�����Ӧѡ����ʽ�ζ��ܣ�
��3����ˮ�����������ʱ����Ͳ�ڵ�Һ��Ҫ��ˮ����Һ����ƽ�����ý���dz��³�ѹ�µ����������
��4����������һ�����ʵ���Ũ�ȵ���Һ��Ҫ���������ش�
��5��������ؾ��������ԣ�˫��ˮ���л�ԭ�ԣ����߿��Է�����������Ӧ��������ؾ�����ɫ��
��6���������ƵĴ���=
 ��100%��
��100%��������������һ�����������⣬����ѧ�������ͽ��������������ۺ��Խ�ǿ���ѶȺܴ�

��ϰ��ϵ�д�
 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ
���й����Ƶ��������������ȷ���ǣ�������
| A��Na2O�����ں������ | B��Na2O���ȶ����ܼ�����O2��������Na2O2 | C��Na2O2�ǰ�ɫ���壬����ˮ���õõ�O2��NaOH | D��Na2O2��ˮ�ķ�Ӧ�У���������Na2O2����ԭ����ˮ |
���й����Ƶ��������������ȷ���ǣ�������
| A��Na2O�����ں������ | B��Na2O���ȶ����ܼ�����O2��������Na2O2 | C��Na2O2�ǰ�ɫ���壬����ˮ���õõ�O2��NaOH | D��Na2O2��ˮ�ķ�Ӧ�Ǹ��ֽⷴӦ |
