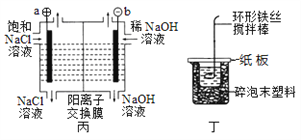
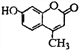
��Ŀ����
����Ŀ����NAΪ�����ӵ�����ֵ�������й�������ȷ����
A. 22g N2O��CO2��ɵĻ����������������ԭ������Ϊ1��5NA
B. 0��1mol��L-1�İ�ˮ��Һ�У�NH3��H2O��NH4+����Ŀ֮��Ϊ0.1NA
C. һ��������Fe����ϡ�����У�������22.4L NOʱ��ת�Ƶ�����Ϊ3NA
D. ������78g��������Ϊ26������Ȳ�ı���Һ�У�����̼ԭ�ӵ���ĿΪ6NA
���𰸡�D
��������A��N2O��CO2��Ħ��������Ϊ44g/mol����22g���������ʵ���Ϊ0.5mol�������߾�Ϊ��ԭ�ӷ��ӣ���0.5mol������к�1.5NA��ԭ�ӣ���������ԭ��������A����B. δע����Һ�������������0��1mol��L-1�İ�ˮ��Һ��NH3��H2O��NH4+����Ŀ֮�ͣ���B����C. Ϊע���Ƿ�Ϊ��״������ȷ��22.4L NO�����ʵ�������C����D. ��Ȳ�ͱ������ʽ����CH��������78g��Ȳ�ı���Һ�к���CH�����ʵ���Ϊ![]() =6mol������̼ԭ�ӵ���ĿΪ6NA����D��ȷ����ѡD��
=6mol������̼ԭ�ӵ���ĿΪ6NA����D��ȷ����ѡD��

 ÿ��10���ӿ�����������������ϵ�д�
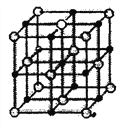
ÿ��10���ӿ�����������������ϵ�д�����Ŀ���±�ΪԪ�����ڱ���ͬ�ڵ�һ���֣� A��B��C��D��E����Ԫ�������ڱ��е�λ������ͼ��ʾ��CԪ�ص�ԭ������������Ϊ������3����
A | E | C | |
B | D |
�ش��������⣺
��1��BԪ�������ڱ��е�λ��Ϊ__________��
��2��D������������Ӧˮ����Ļ�ѧʽΪ__________��
��3��������ʵ��˵��CԪ�صķǽ����Ա���Ԫ�صķǽ�����ǿ����__________��
a��C������H2S��Һ��Ӧ����Һ�����
b����������ԭ��Ӧ�У�1molC���ʱ�1molS�õ��Ӷ�
c��C��S��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
d. CԪ�صļ��⻯��е����SԪ�صļ��⻯��
��4��B��D��Ԫ�صĵ��ʷ�Ӧ���ɻ�����M�����Ľṹ���Ƽ��飬д��M�ĵ���ʽ_____��
��5��A��þ�γɵ�1mol������N��ˮ��Ӧ������2molMg(OH)2��1mol��̬��������������̼��������Ϊ9:1��д��N��ˮ��Ӧ�Ļ�ѧ����ʽ______________________________��
��6��ͭ��һ��Ũ�ȵ����������Ļ���ᷴӦ�����ɵ���ֻ������ͭ��ͬʱ���ɵ�����������ɱ�������Ԫ����ɣ��������Է���������С��50��Ϊ��ֹ��Ⱦ����������������ȫת��Ϊ��ۺ������Σ�����1L2.2mol/LNaOH��Һ��1molO2������������ķ���ʽ�����ʵ����ֱ�Ϊ__________��