ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ‘ΣΥΊ÷ήΤΎ±μΫ“ ΨΝΥ–μΕύ‘ΣΥΊΒΡœύΥΤ–‘ΚΆΒί±δΙφ¬…Θ§œ¬±μ÷–Υυ ΨΒΡ“Μ–©‘ΣΥΊΓΘ

Θ®1Θ©–¥≥ωΔό‘ΣΥΊΒΡάκΉ”ΒΡΚΥΆβΒγΉ”≈≈≤Φ Ψ“βΆΦ____________________ΓΘ
Θ®2Θ©ΗχΔήΔίΔόΔΏΔύ–Έ≥…ΒΡΒΞΚΥάκΉ”ΒΡΑκΨΕ¥”¥σΒΫ–Γ≈≈–ρΘΚ_________Θ®–¥άκΉ”ΖϊΚ≈Θ©ΓΘ
Θ®3Θ©ΗχΔΌΔΎΔέΔήΔίΔόΔΏΔύΔαΒΡ‘≠Ή”ΑκΨΕ¥”¥σΒΫ–Γ≈≈–ρΘΚ__________Θ®–¥‘≠Ή”ΖϊΚ≈Θ©ΓΘ
Θ®4Θ©ΗχΔΎΔόΔαΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΦν–‘¥”¥σΒΫ–Γ≈≈–ρ__________Θ®–¥Μ·―ß ΫΘ©ΓΘ
Θ®5Θ©ΗχΔέΔήΔίΒΡΖ«Ϋπ τ–‘¥”¥σΒΫ–Γ≈≈–ρ_________Θ®–¥‘ΣΥΊΖϊΚ≈Θ©ΓΘ
Θ®6Θ©Δέ «“Μ÷÷”ΟΆΨ °Ζ÷ΙψΖΚΒΡΖ«Ϋπ τΘ§«κ–¥≥ωΔέΒΡΉνΗΏΦέ―θΜ·Έο”κΙΐΝΩΒΡΔαΒΡ«β―θΜ·ΈοΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ___________________________________ΓΘ
Θ®7Θ©ΔΏΚ≈‘ΣΥΊΒΡ«β―θΜ·Έο”–ΧΊ βΒΡΜ·―ß–‘÷ Θ§–¥≥ωΔΏΚ≈‘ΣΥΊΒΡ¬»Μ·Έο”κΉψΝΩΒΡΔόΚ≈‘ΣΥΊΒΡ«β―θΜ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ___________________________________ΓΘ
ΓΨ¥πΑΗΓΩ 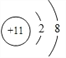 Cl->O2->F->Na+>Al3+ K> Na> Al> Li> Cl> C> O> F> H KOH> NaOH> LiOH F>O>C CO2+2KOH=K2CO3+H2O Al3+ ΘΪ4 OH- = Al02- ΘΪ 2 H2O
Cl->O2->F->Na+>Al3+ K> Na> Al> Li> Cl> C> O> F> H KOH> NaOH> LiOH F>O>C CO2+2KOH=K2CO3+H2O Al3+ ΘΪ4 OH- = Al02- ΘΪ 2 H2O
ΓΨΫβΈωΓΩΘ®1Θ©–¥≥ωΔόΈΣΡΤ‘ΣΥΊΘ§άκΉ”ΒΡΚΥΆβΒγΉ”≈≈≤Φ Ψ“βΆΦ ΘΜΘ®2Θ©ΨΏ”–œύΆ§ΒγΉ”≤ψΫαΙΙΒΡάκΉ”Θ®ΒΞΚΥΘ©,ΚΥΒγΚ… ΐ‘Ϋ–Γ,ΑκΨΕ‘Ϋ¥σ,’βάο“≤÷Μ”–“θάκΉ”ΑκΨΕ¥σ”Ύ―τάκΉ”ΑκΨΕΖϊΚœ,»γ―θάκΉ”ΜρΖζάκΉ”ΑκΨΕ>ΡΤάκΉ”ΜρΟΨάκΉ”Μρ¬ΝάκΉ”,ΒΪ «Φ«ΉΓ―θάκΉ”ΑκΨΕ>ΖζάκΉ”,ΡΤάκΉ”>ΟΨάκΉ”,”κ‘≠Ή”ΑκΨΕΥ≥–ρ“Μ÷¬Θ§ Ι Cl->O2->F->Na+>Al3+ ΘΜΘ®3Θ©Ά§“Μ÷ήΤΎ¥”ΉσΒΫ”“,ΑκΨΕ“ά¥ΈΦθ–ΓΘ§Ά§÷ςΉε¥”…œΒΫ–ΓΑκΨΕ“ά¥Έ‘ω¥σΘ§ΔΌΔΎΔέΔήΔίΔόΔΏΔύΔαΒΡ‘≠Ή”ΑκΨΕ¥”¥σΒΫ–Γ≈≈–ρΘΚK> Na> Al> Li> Cl> C> O> F> H ΘΜΘ®4Θ©Ά§“ΜΉε÷–,¥”…œΒΫœ¬,‘ΣΥΊΉνΗΏΦέ―θΜ·ΈοΥυΕ‘”ΠΒΡΥ°Μ·ΈοΒΡΦν–‘‘ω«ΩΘ®Υα–‘Φθ»θΘ©Θ§ΔΎΔόΔαΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΦν–‘¥”¥σΒΫ–Γ≈≈–ρ KOH> NaOH> LiOHΘΜΘ®5Θ©Ά§÷ήΤΎ‘ΣΥΊ,Ή‘Ήσœρ”“,‘ΣΥΊΒΡΖ«Ϋπ τ–‘“ά¥Έ‘ω«Ω,ΔέΔήΔίΒΡΖ«Ϋπ τ–‘¥”¥σΒΫ–Γ≈≈–ρF>O>CΘΜΘ®6Θ©Δέ «“Μ÷÷”ΟΆΨ °Ζ÷ΙψΖΚΒΡΖ«Ϋπ τΘ§«κ–¥≥ωΔέΒΡΉνΗΏΦέ―θΜ·Έο”κΙΐΝΩΒΡΔαΒΡ«β―θΜ·ΈοΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ CO2+2KOH=K2CO3+H2OΘΜΘ®7Θ©ΔΏΚ≈‘ΣΥΊΒΡ«β―θΜ·Έο”–ΧΊ βΒΡΜ·―ß–‘÷ Θ§–¥≥ωΔΏΚ≈‘ΣΥΊΒΡ¬»Μ·Έο”κΉψΝΩΒΡΔόΚ≈‘ΣΥΊΒΡ«β―θΜ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫAl3+ ΘΪ4 OH- = Al02- ΘΪ 2 H2OΓΘ
ΘΜΘ®2Θ©ΨΏ”–œύΆ§ΒγΉ”≤ψΫαΙΙΒΡάκΉ”Θ®ΒΞΚΥΘ©,ΚΥΒγΚ… ΐ‘Ϋ–Γ,ΑκΨΕ‘Ϋ¥σ,’βάο“≤÷Μ”–“θάκΉ”ΑκΨΕ¥σ”Ύ―τάκΉ”ΑκΨΕΖϊΚœ,»γ―θάκΉ”ΜρΖζάκΉ”ΑκΨΕ>ΡΤάκΉ”ΜρΟΨάκΉ”Μρ¬ΝάκΉ”,ΒΪ «Φ«ΉΓ―θάκΉ”ΑκΨΕ>ΖζάκΉ”,ΡΤάκΉ”>ΟΨάκΉ”,”κ‘≠Ή”ΑκΨΕΥ≥–ρ“Μ÷¬Θ§ Ι Cl->O2->F->Na+>Al3+ ΘΜΘ®3Θ©Ά§“Μ÷ήΤΎ¥”ΉσΒΫ”“,ΑκΨΕ“ά¥ΈΦθ–ΓΘ§Ά§÷ςΉε¥”…œΒΫ–ΓΑκΨΕ“ά¥Έ‘ω¥σΘ§ΔΌΔΎΔέΔήΔίΔόΔΏΔύΔαΒΡ‘≠Ή”ΑκΨΕ¥”¥σΒΫ–Γ≈≈–ρΘΚK> Na> Al> Li> Cl> C> O> F> H ΘΜΘ®4Θ©Ά§“ΜΉε÷–,¥”…œΒΫœ¬,‘ΣΥΊΉνΗΏΦέ―θΜ·ΈοΥυΕ‘”ΠΒΡΥ°Μ·ΈοΒΡΦν–‘‘ω«ΩΘ®Υα–‘Φθ»θΘ©Θ§ΔΎΔόΔαΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΦν–‘¥”¥σΒΫ–Γ≈≈–ρ KOH> NaOH> LiOHΘΜΘ®5Θ©Ά§÷ήΤΎ‘ΣΥΊ,Ή‘Ήσœρ”“,‘ΣΥΊΒΡΖ«Ϋπ τ–‘“ά¥Έ‘ω«Ω,ΔέΔήΔίΒΡΖ«Ϋπ τ–‘¥”¥σΒΫ–Γ≈≈–ρF>O>CΘΜΘ®6Θ©Δέ «“Μ÷÷”ΟΆΨ °Ζ÷ΙψΖΚΒΡΖ«Ϋπ τΘ§«κ–¥≥ωΔέΒΡΉνΗΏΦέ―θΜ·Έο”κΙΐΝΩΒΡΔαΒΡ«β―θΜ·ΈοΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ CO2+2KOH=K2CO3+H2OΘΜΘ®7Θ©ΔΏΚ≈‘ΣΥΊΒΡ«β―θΜ·Έο”–ΧΊ βΒΡΜ·―ß–‘÷ Θ§–¥≥ωΔΏΚ≈‘ΣΥΊΒΡ¬»Μ·Έο”κΉψΝΩΒΡΔόΚ≈‘ΣΥΊΒΡ«β―θΜ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫAl3+ ΘΪ4 OH- = Al02- ΘΪ 2 H2OΓΘ

ΓΨΧβΡΩΓΩΡ≥―ßœΑ–ΓΉιΈΣΧΫΨΩMnO2”κFeCl3ΓΛ6H2OΒΡΖ¥”ΠΘ§…ηΦΤΝΥœ¬Ν–ΉΑ÷ΟΘΚ

≤ι‘ΡΈΡœΉ”–»γœ¬–≈œΔΘΚΔΌFeCl3 »έΒψ282ΓφΘ§Ζ–Βψ315Γφ, 2FeCl3 ![]() 2FeCl2+Cl2Γϋ
2FeCl2+Cl2Γϋ
ΔΎFeCl6ΓΛ6H2O »έΒψ37ΓφΘ§Ζ–Βψ285Γφ
Β―ιΙΐ≥ΧΦ«¬ΦΘΚ
≤ΌΉς≤Ϋ÷η | œ÷œσ |
1.Φλ≤ιΉΑ÷ΟΤχΟή–‘Θ§ΧμΦ”œύ”Π“©ΤΖΘ§ Βψ»ΦΨΤΨΪΒΤΘ§ΩΣ ΦΗχ ‘ΙήAΦ”»» | |
2.¥ρΩΣaΘ§ΙΊ±’bΓΔcΘ§ ”Ο Σ»σΒΡάΕ…Ϊ ·»ο ‘÷ΫΧυΫϋaΩΎ | A÷–≥ωœ÷ΑΉΈμΘ§άΕ…Ϊ ·»ο ‘÷Ϋ±δΚλ |
3.¥ρΩΣbΘ§ΙΊ±’aΓΔc | A÷–÷πΫΞ≤ζ…ζΜΤ…ΪΤχΧεΘ§ ‘ΙήΦΑΒΦΙή±ΎΡΎΗΫ”–ΜΤ…Ϊ“ΚΒΈΘ§B÷–»ή“Κ±δΚλ |
4.¥ρΩΣcΘ§ΙΊ±’aΓΔb | C÷–»ή“Κ±δΉΊΜΤ…Ϊ |
5.ΆΘ÷ΙΦ”»» |
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©A÷–ΒΡΑΉΈμ «________________________ΓΘ
Θ®2Θ©Ιή±ΎΡΎ–Έ≥…ΜΤ…Ϊ“ΚΒΈΘ§Ω…ΡήΒΡ‘≠“ρ «______________________ΓΘ
Θ®3Θ©B÷–»ή“Κ±δΚλΘ§«κ”ΟάκΉ”ΖΫ≥Χ ΫΫβ ΆΤδ‘≠“ρ_______________________ΓΘ
Θ®4Θ©Ε‘C÷–»ή“Κ±δΜΤ…ΪΘ§–ΓΉι’ΙΩΣΫχ“Μ≤Ϋ Β―ιΘΚ

ΔώΘ°C÷–±δΜΤΒΡ‘≠“ρ «___________________________________________ΓΘ
ΔρΘ°C÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «________________________________ΓΘ
Θ®5Θ©Ε‘A÷–≤–ΝτΈοΫχ––Ζ÷άκΘ§Τδ÷–ΒΟΒΫ“Μ÷÷≤Μ»ή”ΎΥ°ΒΡΚλΉΊ…ΪΙΧΧεΘ§–¥≥ωA÷–…ζ≥…ΗΟΙΧΧεΒΡΜ·―ßΖΫ≥Χ Ϋ____________________________________ΓΘ
Θ®6Θ©‘ΎA÷–MnO2ΖΔ…ζΒΡΜ·―ßΖ¥”ΠΈΣ__________________________________ΓΘ
ΓΨΧβΡΩΓΩ1,2-Εΰδε““ΆιΩ…ΉςΤϊ”ΆΩΙ±§ΦΝΒΡΧμΦ”ΦΝΘ§œ¬ΆΦ « Β―ι “÷Τ±Η1,2-“ΜΕΰδε““Άι≤ΔΫχ––“ΜœΒΝ–œύΙΊ Β―ιΒΡΉΑ÷ΟΘ®Φ”»»ΦΑΦ–≥÷…η±Η“―¬‘Θ©ΓΘ

”–ΙΊ ΐΨίΝ–±μ»γœ¬ΘΚ
““¥Φ | 1,2-Εΰδε““Άι | ““Ο― | |
Ή¥Χ§ | Έό…Ϊ“ΚΧε | Έό…Ϊ“ΚΧε | Έό…Ϊ“ΚΧε |
ΟήΕ»/g/cm3 | 0.79 | 2.2 | 0.71 |
Ζ–Βψ/Γφ | 78.5 | 132 | 34.6 |
»έΒψ/Γφ | -130 | 9 | -116 |
«κΑ¥“Σ«σΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ© Β―ιΩΣ Φ÷°«Α±Ί“ΣΒΡ≤ΌΉς «____________ΓΘ
Θ®2Θ©“«ΤςAΒΡΟϊ≥ΤΈΣ____________ΓΘ
Θ®3Θ© Β―ιΙΐ≥Χ÷–Θ§»τΖΔœ÷ΉΑΘ§B÷–Υ°―ΊΒΦΙήG…œ…ΐΘ§‘ρ–ηΫχ––ΒΡ≤ΌΉς «____________ΓΘ
Θ®4Θ©ΉΑ÷ΟD÷–ΤΖΚλ»ή“ΚΒΡΉς”Ο «____________ΓΘ
Θ®5Θ©Ζ¥”ΠΙΐ≥Χ÷–”Π”ΟάδΥ°ά以ΉΑ÷ΟEΘ§Τδ÷ς“ΣΡΩΒΡ «____________ΓΘ
Θ®6Θ©≈–ΕœΗΟ÷Τ±ΗΖ¥”Π“―Ψ≠Ϋα χΒΡΉνΦρΒΞΖΫΖ® «____________ΘΜΫαΙϊ―ß…ζΖΔœ÷Ζ¥”ΠΫα χ ±Θ§ΈόΥ°““¥ΦœϊΚΡΝΩ¥σ¥σ≥§Ιΐάμ¬έ÷ΒΘ§Τδ‘≠“ρ «____________Θ®¥π≥ωΤδ÷–ΝΫΧθΦ¥Ω…Θ©ΓΘ
