��Ŀ����
�ס�������̽����ѧϰС�飬������ⶨ����������Ʒ����������Na2O���Ĵ��ȡ�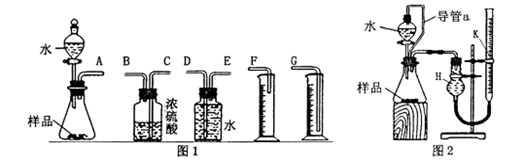
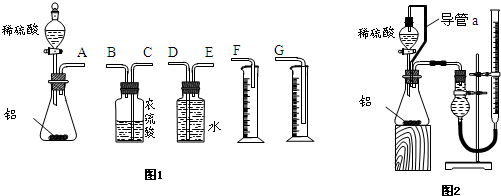
��1������ͬѧ��ѡ��ͼ1ʵ��װ�����ʵ�飺
��д��ʵ������������Ӧ�Ļ�ѧ����ʽ ��
�ڸ���ͬѧѡ�����װ�õ�����˳���ǣ�
A�ӣ� ������ ���ӣ� ������ ���ӣ� ������ӿ���ĸ���ɲ���������
��2�� ����ͬѧ��ϸ��������ͬѧ��ʵ��װ�ú���Ϊ��ˮ������ƿ�У���ʹ������������ Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��;ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС���������������ͼ2��ʾ��ʵ��װ�á�
��װ���е���a�������ǣ� ��
��ʵ�������,�ڶ�ȡ������k��Һ�����ʱ������Ϊ�������˳���� (��A�� B��C��ĸ��ʾ)
A. ������������k�а�Һ�����͵���ƽ
B. �ȴ�ʵ��װ����ȴ
C. �����ƶ�������k,ʹk��Һ����H�е�Һ����ƽ
����ʵ������Ʒ������Ϊwg��ʵ��ǰ��������k��Һ������ֱ�ΪV1��V2(����ɱ����������Ʒ�Ĵ���Ϊ_______ (��w��V�ı���ʽ����
��1���� Na2O+H2O=2NaOH��2Na2O2+2H2O=4NaOH+O2����2�֣�����EDG ��1�֣�
��2���ٿ���ʹ��Һ©���������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱˮ��˳�����£��������ڼ���ˮ��������������1�֣���BCA ��1�֣�
��Na2O2%=195��(V1-V2)/28w��100%��2�֣���
���������������1������ͬѧѡ��ͼ1ʵ��װ�����ʵ�飺��ʵ����������Ӧ�Ļ�ѧ����ʽΪ��Na2O+H2O=2NaOH��2Na2O2+2H2O=4NaOH+O2������ʵ�������������������Բ����ų�ˮ������ķ������ⶨ�����Ը���ͬѧѡ�����װ�õ�����˳����A��E��D��G�����Բ���Ũ����������������Ϊ���õ�����ˮ������2��ˮ������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���a�����þ�������������һȱ�㣬��a�������ǿ���ʹ��Һ©���������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱˮ��˳�����£��������ڼ���ˮ��������������ʵ�������,�ڶ�ȡ������k��Һ�����ʱ��Ӧ�õȴ�ʵ��װ����ȴ��Ȼ�������ƶ�������k,ʹk��Һ����H�е�Һ����ƽ��������Ҫ��������������k�а�Һ�����͵���ƽ���ʺ�����˳��ΪBCA������ʵ������Ʒ������Ϊwg��ʵ��ǰ��������k��Һ������ֱ�ΪV1��V2(����ɱ�����������������������Ϊ(V1-V2)mL������2Na2O2+2H2O=4NaOH+O2������������������Ϊ32��(V1-V2)��156/22.4g�����Թ������Ƶ�����m=156��(V1-V2)/22.4������Ʒ�Ĵ���Ϊ��156��(V1-V2)/22.4��100%������Ϊ195��(V1-V2)/28w��100%��
���㣺�Ƽ��仯����
���������⿼�����Ƽ��仯��������ʣ��ÿ����Ǹ߿�������ص���ѵ㣬������ؿ���ѧ����ʵ��ķ��������ͼ��������������Ѷ��еȡ�

ij��ѧ�мס�������̽����ѧϰС�飬��������С��������ͭ�Ͻ���������ϡ���ᷴӦ�ⶨͨ��״����Լ20�桢1.01��105Pa���µ�����Ħ�������Vm����

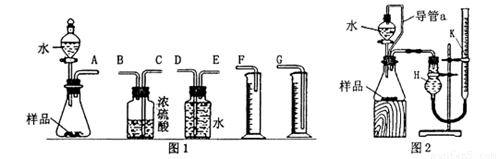
��һ������ͬѧ�������ͼ1��ʾ��װ�������ʵ�顣
��1��д��װ��I�з�����Ӧ�����ӷ���ʽ�� ��
��2��ʵ�鿪ʼʱ���ȴ�Һ©���ϿڵIJ������������������������һ�������ϡ�����˳��������ƿ�С���������Ƿ�����ԭ�� ��
��3��ʵ�����ʱ������������������Ƶ��ڣ� ��
��4����ƿ�вд��������ʵ�����Ƿ���Ӱ�죺 ����С�����û�С��������жϡ������������ɣ� ��
����������ͬѧ��ϸ�����˼���ͬѧ��ʵ��װ�ú���Ϊ��ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�еĿ����ų���ʹ�������������ƫ��ʵ����������ӹ��ƿ
����Ͳ�ĵ�����������ˮ���ڣ�ʹ�������������ƫС�����������������ͼ2��ʾ��ʵ��װ�á�
��1��װ���е���a�������ǣ� ��
��2��ʵ����ȷ�ⶨ��4�����ݣ����±���
|
|
ʵ��ǰ |
ʵ��� |
|
��ͭ�Ͻ�������g�� |
m1 |
m2 |
|
��Һ�ܣ�C�������mL�� |
V1 |
V2 |
�����������ݼ���ͨ��״���µ�����Ħ�������Vm= ��