��Ŀ����
����ѧ����ѡ��5���л���ѧ������ ��15�֣�
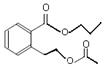
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���ú�D����Է���������ȣ���EΪ��֧���Ļ����
������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ����C�����еĹ����������� ______________��������B���ܷ����ķ�Ӧ�ǣ�����ĸ��ţ���______________
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ
d��������Ӧ e��ˮ�ⷴӦ f���û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��__________________��
��3����Ӧ��ʵ���м��ȵ�Ŀ���ǣ� .��
��4��A�Ľṹ��ʽ�� __________________��
��5��д��ͬʱ������������������B��ͬ���칹������ͬ���칹��Ľṹ��ʽ��
���м��ȡ�������ṹ
�����ڷ������γɵ���
���� FeCl3��Һ������ɫ��Ӧ
��1���Ȼ� ��2�֣�
e ��2�֣�
��2��CH3COOH+CH3CH2CH2OH CH3COOCH2CH2CH3+H2O��2�֣�
CH3COOCH2CH2CH3+H2O��2�֣�
��3���ټӿ췴Ӧ����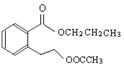
 �ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�
�ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�

������������������п�֪��C�ܸ�NaHCO3������Ӧ������C�����Ȼ����ٴ�C��D�ķ�Ӧ�������Կ���������һ��������Ӧ�����DΪ��������Ϊ�ú�D����Է���������ȣ�����D���ִ�Ϊ��C��һ��̼ԭ�ӵĴ�����EΪ��֧���Ļ�������������ʹ�����ֱ���ġ�
E�е�̼ԭ����ĿΪ��
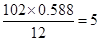 ����ԭ����Ϊ��
����ԭ����Ϊ��
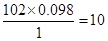 �����ʽΪ��C5H10O2
�����ʽΪ��C5H10O2
Ҳ����˵EΪ������������ǿɵ����½⣺
��1���Ȼ� e
��2��CH3COOH+CH3CH2CH2OH CH3COOCH2CH2CH3+H2O��2�֣�
CH3COOCH2CH2CH3+H2O��2�֣�
��3���ټӿ췴Ӧ����
�ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�

���㣺�����л���֮����ת����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���֪ijһ��ȼ�Ϻ�̼�����������Ԫ�أ�Ϊ�ⶨ��ȼ�ϵ���ɣ�����ȼ�Ϸ��뵽����������ȼ�գ���ʹ������CO2��H2O�����Լ�ʣ���O2ȫ��ͨ������ͼ��ʾ��װ�ã��õ����±����г���ʵ�����ݣ��������ɵ�������ȫ�������գ���
| | ʵ��ǰ | ʵ��� |
| ������ / g | 101��1 | 103��8 |
| �ҵ����� / g | 82��0 | 86��4 |
��1����ȼ�Ϸ�����̼����ԭ�ӵ���Ŀ��Ϊ_______��
��2����֪��ȼ�Ϸ��ӵ�ʽ��Ϊ46����ÿ�������к���1����ԭ�ӣ��������ʽΪ________________��
��15�֣�
���������������ҩ�Ϳ�ϵ�����Ӧ�ù㷺��
��1�����й��ڻ�����I��˵������ȷ���� 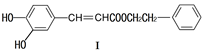
| A����FeCl3��Һ��������ɫ |
| B�����ܷ���������Ӧ��������Ӧ |
| C�������巢��ȡ���ͼӳɷ�Ӧ |
| D��1mol������I�����2molNaOH��Ӧ |

������II�ķ���ʽΪ ��1mol������II���� molH2ǡ�÷�Ӧ���ɱ���������
��3��������II���ɷ����廯����III��IV�ֱ�ͨ����ȥ��Ӧ��á���ֻ��III����Na��Ӧ����H2��III�Ľṹ��ʽΪ ��д1�֣�����IV����II�ķ�Ӧ����Ϊ ��
��4���ۺ���
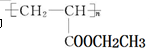 �������Ʊ�Ϳ�ϡ��䵥��Ľṹ��ʽΪ ���������Ʒ�Ӧ�ٵķ���������ϩΪ�л�ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ ��
�������Ʊ�Ϳ�ϡ��䵥��Ľṹ��ʽΪ ���������Ʒ�Ӧ�ٵķ���������ϩΪ�л�ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ �� 





