��Ŀ����
����˵����ȷ���ǣ� ��
A��þ�뼫ϡ���ᷴӦ��������淋����ӷ���ʽΪ:4Mg+6H++N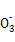 =4Mg2++N =4Mg2++N +3H2O +3H2O |
| B��������,0.1 mol/LһԪ��HA��Һ��c(OH-)/c(H+)=1��10-8,�����Һ��pH=3 |
| C����ͬ������,Ũ�Ⱦ�Ϊ0.01 mol/L��NH4Cl��Һ��NaCl��Һ��,ǰ�ߵ���������Ũ�ȴ��ں��ߵ���������Ũ�� |
| D�����ʵ���Ũ����ȵĴ��������������Һ�������Ϻ����Һ��:c(Na+)+c(H+)=c(CH3COO-)+c(OH-)+c(CH3COOH) |
B
����

��ϰ��ϵ�д�
�����Ŀ
�ס��ҡ�����������������ˮ�����ʣ��ֱ���NH4+��Ba2����Mg2����H����OH���� Cl����HCO3-��SO42-�еIJ�ͬ�����Ӻ������Ӹ�һ����ɣ�������Һ�ֱ��������������ʵ���Һ��ϣ����а�ɫ�������ɣ����Ϊ
| A��MgSO4 | B��Ba(HCO3)2 | C��Mg(HCO3)2 | D��Ba(OH)2 |
�������ӷ���ʽ��д��ȷ����
| A������ʯ��ˮ�������С�մ���Һ��Ӧ��Ca2����OH����HCO3��=CaCO3����H2O |
| B��������Һ�еμ�Ba(OH)2��Һ��SO42�Cǡ����ȫ������Al3++Ba2++ SO42�C+3OH�C=BaSO4��+Al(OH)3�� |
| C��200 mL 2 mol��L�C1��FeBr2��Һ��ͨ��11.2 L��״���µ�������4 Fe2++6Br�C+5Cl2=4Fe3++3Br2+ 10Cl�C |
| D����ǿ����Һ�д���������Fe(OH)3��Ӧ����Na2FeO4��3ClO�C+2Fe(OH)3=2FeO42�C+3Cl�C+H2O+4H+ |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
| A����������ˮ��Ӧ��Na+2H2O��Na++2OH��+H2�� |
| B��Cu��Ũ���ᷴӦ��NO2��Cu+4HNO3(Ũ)��Cu2��+2NO3��+2NO2��+2H2O |
| C����Na2SiO3��Һ��ͨ�����CO2��SiO32��+ CO2 +H2O =H2SiO3��+ CO32�� |
| D����NaHSO4��Ba(OH)2��Һ��������ԣ�2H��+SO42��+Ba2��+2OH��= BaSO4��+2H2O |
���н�����ʵ�ķ���ʽ����ȷ����(����)
| A����Ũ������鰱��NH3��HCl=NH4Cl |
B��̼������Һ�Լ��ԣ�CO32����H2O HCO3����OH�� HCO3����OH�� |
| C����������������ʴʱ������������������Fe��3e��=Fe3�� |
| D������ʢ��ʯ��ˮ���Լ�ƿ�ڱڳ��ְ�ɫ���壺Ca(OH)2��CO2=CaCO3����H2O |
�������ӷ���ʽ��д��ȷ����(����)
A������ɫ�ĵ�����Һ��ͨ������SO2������ɫ��Һ:I2+SO2+2H2O 2I-+SO42��+4H+ 2I-+SO42��+4H+ |
B����֪����ƽ�ⳣ��:H2CO3>HClO>HCO32��,��NaClO��Һ��ͨ������������̼:2ClO-+CO2+H2O 2HClO+CO42�� 2HClO+CO42�� |
C��NH4HCO3��Һ�����NaOH��Һ��Ӧ:NH4++OH- NH3��+H2O NH3��+H2O |
D��FeI2��Һ��ͨ�����Cl2:2Fe2++2I-+2Cl2 2Fe3++I2+4Cl- 2Fe3++I2+4Cl- |
�������ӷ���ʽ��ȷ����(����)
| A�����ô����ܽ⺬̼��Ƶ�ˮ����CaCO3��2H��=Ca2����H2O��CO2�� |
B����ͭΪ�缫��ⱥ��ʳ��ˮ��2Cl����2H2O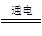 Cl2����H2����2OH�� Cl2����H2����2OH�� |
| C����������Һ�еμӹ�����ˮ��Al3����4NH3��H2O=AlO2����4NH4+��2H2O |
| D����С�մ���Һ�м�����Ba(OH)2��Һ��2HCO3����Ba2����2OH��=BaCO3����2H2O��CO32�� |
�����£����и���������ָ����Һ��һ���ܴ����������(����)
| A�����ܽ�Al2O3����Һ��Na����K����HCO3����NO3�� |
| B��0.1 mol��L��1Ca(ClO)2��Һ��K����Na����I����Cl�� |
| C����ʹ�����Ժ�ɫ����Һ��K����Fe2����Cl����NO3�� |
| D������KSCN�Ժ�ɫ����Һ��Na����Mg2����Cl����SO42�� |
���з�Ӧ�����ӷ���ʽ��ʾ��ȷ���ǣ�������
| A������������ϡ�����У�FeS��2H��=Fe2����H2S�� |
| B��NH4HCO3���ڹ�����NaOH��Һ�У�HCO3-��OH��=CO32-��H2O |
| C��С�մ���Һ�м�����Ba��OH��2��Һ��2HCO3-��Ba2����2OH��=BaCO3����2H2O��CO32- |
| D������������˿Ͷ��ϡ�����У�3Fe��8H����2NO3-=3Fe2����2NO����4H2O |