��Ŀ����
����˵����ȷ����
A�������£����ʵ���Ũ�Ⱦ�Ϊ0.1mol��L?1Na2CO3��NaHCO3�ĵ���������Һ�У�
2c(OH?)��2c(H+)��3c(H2CO3)��c(HCO3��)��c(CO32?)
B����H��0,��S��0�ķ�Ӧ�����Է���Ӧ����H��0,��S��0�ķ�Ӧ�κ��������Ƿ��Է���Ӧ��
C����֪��P4(g)��6Cl2(g)��4PCl3(g) ��H��akJ��mol��1?
P4(g)��10Cl2(g)��4PCl5(g)��H�� bkJ��mol��1
P4������������ṹ��PCl5��P��Cl���ļ���ΪckJ��mol��1,PCl3��P��Cl���ļ���Ϊ1.2ckJ��mol��1���ɴ˼���Cl��Cl���ļ���

D����һ���¶������̶����Ϊ2L�ܱ���������������Ӧ��2SO2(g)+O2(g) 2SO3(g)
2SO3(g)
��H��0����v(SO2)= v(SO3)ʱ��˵���÷�Ӧ�Ѵﵽƽ��״̬
A
��������
���������A�����������غ㣬����Na2CO3��Һ��c(OH?)=c(H+)+2c(H2CO3)��c(HCO3��)������NaHCO3��Һ��c(OH?)+c(CO32?)��c(H+)+c(H2CO3)�����ߵ�Ũ�ȡ��������Ϻ�����Һ���������ʵ����ʵ����������������������ʽ������[c(OH?)=c(H+)+2c(H2CO3)��c(HCO3��)]+[ c(OH?)+c(CO32?)��c(H+)+c(H2CO3)]������������2c(OH?)��2c(H+)��3c(H2CO3)��c(HCO3��)��c(CO32?)����ȷ��B����H��0����S��0 �ڽϸ��¶������Է���������C����������Ӧʽ�ֱ���Ϊ��������(�� - ��)��4�ɵã�PCl3(g)+Cl2(g)= PCl5(g) ����H�� ���ɣ�
���ɣ�
PCl3(g)??? +??? Cl2(g)??? =????? PCl5(g)
��1��P��Cl��?? ��1��Cl��Cl��?? ��1��P��Cl��
3��1.2c???????? Q????????????? ??????????? 5��c
(3.6c + Q -5c)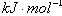 =
= �� Q=
�� Q= ����C����
����C����
Dѡ���ͬ���ʱ�ʾ���ʣ�����ƽ��ʱ����������֮�ȵ��ڻ�ѧ������֮�ȣ��磬V����SO2����V�棨SO3��= 2 : 2����V����SO2��=V�棨SO3��������v(SO2)=v(SO3)δָ����Ӧ���ʵķ��ʴ���
���㣺���黯ѧ��Ӧ�����뻯ѧƽ�⣬�������Һ�Ȼ�������

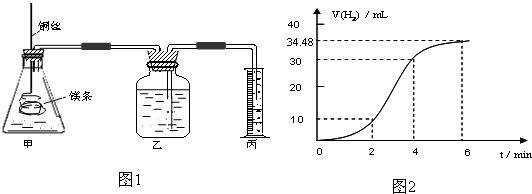
��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ�����
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
| ʵ���� | �� | �� | �� | �� |
| 10%H2O2�����/mL | 5.0 | 5.0 | V1 | V2 |
| 20%��������/mL | 0 | 0.5 | 1.0 | V3 |
| ˮ�����/mL | 15 | 14.5 | V4 | 13.5 |
| ����ʱ��t/s | t1 | t2 | t3 | t4 |
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬��������
����ʵ����t2��t3��t4����ɵó���ʵ�������


 �ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�
�ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�