��Ŀ����
����Ŀ��VA��ĵ����ס��飨As����Ԫ�صĻ������ڿ��к���������������Ҫ��;����ش��������⡣
(1)��Ļ�̬ԭ�ӵĵ����Ų�ʽΪ___________________��
(2)ԭ�ӵĵ�һ��������ָ��̬�����Ի�̬ԭ��ʧȥһ������ת��Ϊ��̬��̬����������Ҫ�����������N��P��Asԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ______________��
(3)NH3�ķе��PH3�ߣ�ԭ����___________��PO43-���ӵ����幹��Ϊ___________��
(4)AsH3����ɫ���д�����ζ�����壬��AsH3��Asԭ�ӵ��ӻ��������Ϊ______________��
(5)H3AsO4��H3AsO3��������ֺ����ᣬ����ݽṹ�����ʵĹ�ϵ������H3AsO4��H3AsO3 ����ǿ��ԭ��_____________________��
(6)��һ�ֵ��ʰ��ף�P4�����ڷ��Ӿ��壬�侧���ṹ����ͼ����֪������������Ӽ�ľ���Ϊ a pm��1pm=10-12m���������ӵ�������ֵΪNA����þ�����ܶ�Ϊ__________________g/cm3��ֻҪ������ʽ�����ؼ��㣩��

���𰸡�1s22s22p63s23p63d104s24p3 N>P>As NH3���Ӽ���ڽ�ǿ���������PH3���Ӽ���н����ķ��»��� �������� sp3�ӻ� H3AsO4��H3AsO3�ɱ�ʾΪ(HO)3AsO��(HO)3As��H3AsO3�е�AsΪ+3�ۣ���H3AsO4�е�AsΪ+5�ۣ������Ը��ߣ�����As-O-H��O�ĵ��Ӹ���Asƫ�ƣ��������H+ ![]()
��������
(1)ȷ������Ԫ�����ڱ��е�λ�ã�Ȼ����ݹ���ԭ��д�������Ų�ʽ��
(2)����ͬ�����һ�����ܵı仯��������
(3)NH3���Ӽ���������PO43-���ӵ����幹�Ϳ��ɼ۲���ӶԻ������ۼ�����ã�
(4)���AsH3����ԭ�ӵļ۲���Ӷ��������ɸ��ݼв���Ӷ������ӻ����͵Ķ�Ӧ��ϵ�ó��𰸣�
(5)���ֺ����ἴ�ɸ�дΪ(HO)mROn��nԽ��R������Խ������Խǿ��
(6)������˾������а���������Ȼ�����ݦ�=![]() ���㡣
���㡣
(1)��Ԫ����33��Ԫ�أ�λ��Ԫ�����ڱ��ĵ������ڵ�VA�壬��̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p3��
(2)ͬ����Ԫ�����϶�������ԭ�Ӱ뾶���������Ե�һ���������ͣ����N��P��Asԭ�ӵĵ�һ�������ɴ�С��˳��ΪN��P��As��
(3)PH3���Ӽ�ֻ���ڷ��»�����NH3���Ӽ�����ڷ��»���֮���������ڱȷ��»�����ǿ���������NH3�ķе��PH3�ߣ�PO43-����ԭ��ΪP�����ЦҼ����Ӷ���Ϊ4������ԭ�ӹµ��Ӷ���Ϊ![]() (5+34��2)=0��PO43-�۲���ӶԶ���Ϊ4+0=4�������幹��Ϊ����������
(5+34��2)=0��PO43-�۲���ӶԶ���Ϊ4+0=4�������幹��Ϊ����������
(4)AsH3����ԭ�ӵļ۲���Ӷ���=3+![]() ������As����sp3�ӻ���
������As����sp3�ӻ���
(5)���ֺ����ἴ�ɸ�дΪ(HO)mROn��H3AsO4�ɱ�ʾΪ(HO)3AsO��H3AsO3�ɱ�ʾΪ(HO)3As��H3AsO4��nֵ��������ǿ������As-O-H��O�ĵ��Ӹ���Asƫ�ƣ��������H+������H3AsO4��H3AsO3����ǿ��
(6)һ�������к��а�������Ϊ��![]() ����һ������������m=
����һ������������m=![]() g��һ�����������V=
g��һ�����������V=![]() =
=![]() ����˾������ܶȦ�=
����˾������ܶȦ�=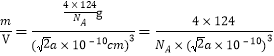 g/cm3��
g/cm3��

����Ŀ��2017�괺�ڣ������ܱ��������������ꡱ���������������������Լ��������������������������Ҫ��ɣ���������������Ⱦ���о�����֮һ��
��1����CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H����574.0 kJ��mol-1
��CH4(g)��4NO(g)��2N2(g)��CO2(g)��2H2O(g) ��H����1160.0 kJ��mol-1
��H2O(g)��H2O(l) ��H����44.0 kJ��mol-1
��д��CH4(g)��NO2(g)��Ӧ����N2(g) ,CO2(g)��H2O(l)���Ȼ�ѧ����ʽ_______________________ ��
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)��2NO(g)![]() N2(g)��CO2(g),ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T0C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)��CO2(g),ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T0C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
Ũ��(mol/L) ʱ��(min) | NO | N2 | CO2 |
0 | 0.100 | 0 | 0 |
10 | 0.058 | 0.021 | 0.021 |
20 | 0.040 | 0.030 | 0.030 |
30 | 0.040 | 0.030 | 0.030 |
40 | 0.032 | 0.034 | 0.017 |
50 | 0.032 | 0.034 | 0.017 |
������˵��������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��־����_______
A������̿������ B��v ��(N2) = 2v�� (NO)
C��������ѹǿ���ֲ��� D�������ڻ��������ܶȱ��ֲ���
E�������ڻ�������ƽ����Է����������ֲ���
F��������CO2��Ũ�ȱ��ֲ���
����T0Cʱ���÷�Ӧ��ƽ�ⳣ��Ϊ______________(С���������λ��Ч����)��
����30 minʱ����ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı��������___________��
����50 minʱ�����¶Ⱥ�������������ٳ���NO��N2��ʹ���ߵ�Ũ�Ⱦ�������ԭ������������ѧƽ��_______(������ƶ������������ƶ������ƶ���)
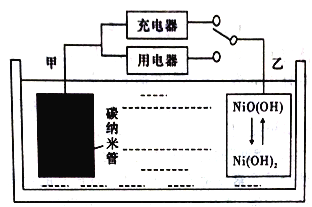
��3�����÷�Ӧ6NO2+8NH3= 7N2+12H2O���ɵ�صķ���������ʵ����Ч��������������ŷţ����ỷ����Ⱦ�����ܳ�����û�ѧ�ܣ�װ����ͼ��ʾ��

��A�缫�ĵ缫��ӦʽΪ______________
�����й��ڸõ�ص�˵����ȷ������_____��
A�����Ӵ��Ҳ�缫�������غ��������缫
B��Ϊʹ��س����ŵ磬���ӽ���Ĥ��ѡ�������ӽ���Ĥ
C����ع���һ��ʱ�䣬��Һ��pH����
D������4.48LNO2������ʱ��ת�Ƶ������ʵ���Ϊ0.8mol