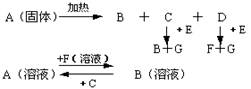
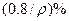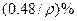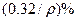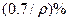��Ŀ����
��̼���ƣ�2Na2CO3��3H2O2���׳ƹ���˫��ˮ����һ�ֺܺõ����������������л��������ڣ������Ϊ��ɫ�����������������Ʊ���������ͼ��ʾ��ͼ��BC��1��BC��2��Ϊ�ȶ���������BC��1��������������Ҵ�����һ��������϶��ɡ�
��̼���Ƽ����Ʊ�����·��
��1���Ʊ������м���BC��1��Ŀ���� ��
��2���ᾧ�����м����Ȼ��ơ����裬������ ��
��3��ϴ�ӳ��˲�Ʒ�����ʵ�ϴ���Լ��� ����ѡ���ţ���
��4��д���Ʊ���̼���Ƶķ���ʽ�� ��
��5����ʵ��ⶨ��Ӧ�¶ȶԲ����Ӱ�����±���
�����ϱ����ݣ�����Ϊ��Ӧ��ѵ��¶�ѡ��ķ�Χ�� ��
 |
��̼���Ƽ����Ʊ�����·��
��1���Ʊ������м���BC��1��Ŀ���� ��
��2���ᾧ�����м����Ȼ��ơ����裬������ ��
��3��ϴ�ӳ��˲�Ʒ�����ʵ�ϴ���Լ��� ����ѡ���ţ���
| A������NaCl��Һ | B��ˮ | C������� | D��̼���Ʊ�����Һ |
��5����ʵ��ⶨ��Ӧ�¶ȶԲ����Ӱ�����±���
| T/��C | �������ٷֺ��� | ���� |
| 5��10 | 13.94 | 85.49 |
| 10��15 | 14.02 | 85.78 |
| 15��20 | 15.05 | 88.38 |
| 20��25 | 14.46 | 83.01 |
��1��2Na2CO3+3H2O2=2Na2CO3��3H2O2��2�֣���2����ǿH2O2���ȶ��ԣ���ֹ��ֽ�
��3������̼���Ƶ��ܽ�ȣ������ڹ�̼�������� ��4�� C ��5��15��20����2�֣�
��3������̼���Ƶ��ܽ�ȣ������ڹ�̼�������� ��4�� C ��5��15��20����2�֣�
��2����˫��ˮ�ֽ⣬���������ȶ�����ǿH2O2���ȶ��ԣ���ֹ��ֽ⣻��4���г�����������������Լ������ܽ��Ʒ

��ϰ��ϵ�д�
�����Ŀ
 ������Һ�����ʵ���������������
������Һ�����ʵ���������������