��Ŀ����
�����Ϊ5L�ĺ��¡������ܱ���������ʼͶ��2mol N2 3mol H2����10s��ƽ�⣬���ƽ��ʱNH3�����ʵ���Ϊ0.8mol������������ʼͶ��a mol N2��b mol H2��ά�ֺ��¡���ѹ��ƽ�⣬���ƽ��ʱNH3�����ʵ���Ϊ1.2mol����ƽ���¶���ͬ����ͬ��ֵ������������ͬ��
��1��������10s ����H2��ʾ��ƽ����Ӧ����v��H2��=______����ƽ��ʱN2��ת����=______��
��2��������Щ��������������Ѵ�ƽ��״̬��______
A�������ҵ������ܶȲ��ٱ仯 B����Ԫ�ص��������ٱ仯
C���������������ʵ��ڰ������������� D������1molN��N��ͬʱ����6mol N-H��
��3����ƽ��ʱ��������ѹǿ______ ��������ѹǿ������ڡ��������ڡ���С�ڡ�����
��4��a=______mol��b=______mol��
��5����ƽ��ʱ�����������Ϊ______L��
��6����ʼʱ��������������ѹǿ��______����
���𰸡��������ɹ�ʽ�÷�Ӧ���ʡ�ת���ʣ����û�ѧƽ��״̬�����ж��Ƿ��ƽ��״̬��������ƽ���¶���ͬ����ͬ��ֵ������������ͬ���ó���������a��b����ֵ�������¶Ȳ��䣬��ѧƽ�ⳣ�����䣬�ó�ƽ��ʱ���������������������״̬���巽�̣��ó���ʼʱ�������������Ĺ�ϵ��
����⣺��1��v��NH3��=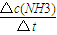 =
=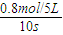 =0.016mol/��L?s��
=0.016mol/��L?s��
v��H2��= v��NH3��=
v��NH3��= ×0.016mol/��L?s��=0.024 mol/��L?s��
×0.016mol/��L?s��=0.024 mol/��L?s��
����N2��=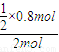 ×100%=20%
×100%=20%
�ʴ�Ϊ��0.024 mol/��L?s�� 20%
��2��A�����¡���ѹ�¶��ڷ�Ӧ N2+3H2=2NH3����������ʵ��������仯�������ҵ��������֮�仯����Ӧ���������������䣮�������ҵ������ܶȲ��ٱ仯��˵�������ҵ�������ٱ仯����Ӧ����ƽ��״̬����A�ԣ�
B����Ԫ�ص�������ʼ���ն��������仯������˵������ƽ��״̬����B����
C��������������ж��Ƿ�ƽ��״̬����ָͬһ���ʵ���������������������ȣ�����������˵���������ɣ�����һ�£�����˵������ƽ��״̬����C����
D����Ӧ����1mol N��N��ͬʱҪ����6molN-H��������N-H��������N-H��������ȣ�����ƽ��״̬����D�ԣ�
��ѡ��A D
��3��������������״̬���̣�PV=nRT����P= ������ƽ���¶���ͬ����ͬ��ֵ������������ͬ����
������ƽ���¶���ͬ����ͬ��ֵ������������ͬ����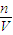 ��ͬ��T��ͬ�����Եó�P��ͬ��
��ͬ��T��ͬ�����Եó�P��ͬ��
���Ա����Ϊ�����
��4��I��N2 +3H2 =2NH3
��ʼ 2mol 3mol 0mol
�仯 0.4mol 1.2mol 0.8mol
ƽ�� 1.6mol 1.8mol 0.8mol
ƽ��ʱN2���������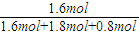 =
=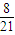
ƽ��ʱH2��������� =
=
ƽ��ʱNH3���������1-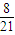 -
- =
=
II�У��������з�Ӧ����������ʵ���Ϊ��n��=1.2mol÷ =6.3mol
=6.3mol
N2 +3H2 �T2NH3
��ʼ amol bmol 0mol
�仯 0.6mol 1.8mol 1.2mol
ƽ�� ��a-0.6��mol ��b-1.8��mol 1.2mol
���ԣ���a-0.6��mol=6.3mol× ��a=3
��a=3
��b-1.8��mol=6.3mol× ��b=4.5
��b=4.5
�ʴ�Ϊ��3 4.5
��5��I��ƽ��ʱN2Ũ�ȣ�c��N2��=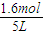
I��ƽ��ʱH2Ũ�ȣ�c��H2��=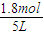
I��ƽ��ʱNH3Ũ�ȣ�c��NH3��=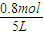
���¶���ƽ�ⳣ��kc=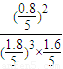 =
=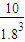 ��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶���ͬƽ�ⳣ����ȣ�
��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶���ͬƽ�ⳣ����ȣ�
���ƽ��ʱ�����������ΪVL����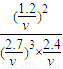 =
=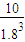 ����V=7.5
����V=7.5
�ʴ�Ϊ��7.5
��6������ʼʱ������ѹǿΪp����ʼʱ������ѹǿp�䣬����������״̬����PV=nRT�ã�
p×5=5RT��p��×7.5=6.3RT������p��=0.84p
�ʴ�Ϊ��0.84
���������⿼���֪ʶ��϶࣬���ʡ�ƽ��״̬�ж��ǿ��Ե��ȵ㣬���û�ѧƽ�ⳣ������������״̬�����ǽ����5����6���ʵĹؼ���
����⣺��1��v��NH3��=
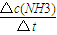 =
=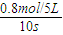 =0.016mol/��L?s��
=0.016mol/��L?s��v��H2��=
 v��NH3��=
v��NH3��= ×0.016mol/��L?s��=0.024 mol/��L?s��
×0.016mol/��L?s��=0.024 mol/��L?s������N2��=
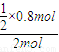 ×100%=20%
×100%=20%�ʴ�Ϊ��0.024 mol/��L?s�� 20%
��2��A�����¡���ѹ�¶��ڷ�Ӧ N2+3H2=2NH3����������ʵ��������仯�������ҵ��������֮�仯����Ӧ���������������䣮�������ҵ������ܶȲ��ٱ仯��˵�������ҵ�������ٱ仯����Ӧ����ƽ��״̬����A�ԣ�
B����Ԫ�ص�������ʼ���ն��������仯������˵������ƽ��״̬����B����
C��������������ж��Ƿ�ƽ��״̬����ָͬһ���ʵ���������������������ȣ�����������˵���������ɣ�����һ�£�����˵������ƽ��״̬����C����
D����Ӧ����1mol N��N��ͬʱҪ����6molN-H��������N-H��������N-H��������ȣ�����ƽ��״̬����D�ԣ�
��ѡ��A D
��3��������������״̬���̣�PV=nRT����P=
 ������ƽ���¶���ͬ����ͬ��ֵ������������ͬ����
������ƽ���¶���ͬ����ͬ��ֵ������������ͬ����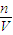 ��ͬ��T��ͬ�����Եó�P��ͬ��
��ͬ��T��ͬ�����Եó�P��ͬ�����Ա����Ϊ�����
��4��I��N2 +3H2 =2NH3
��ʼ 2mol 3mol 0mol
�仯 0.4mol 1.2mol 0.8mol
ƽ�� 1.6mol 1.8mol 0.8mol
ƽ��ʱN2���������
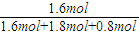 =
=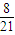
ƽ��ʱH2���������
 =
=
ƽ��ʱNH3���������1-
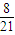 -
- =
=
II�У��������з�Ӧ����������ʵ���Ϊ��n��=1.2mol÷
 =6.3mol
=6.3molN2 +3H2 �T2NH3
��ʼ amol bmol 0mol
�仯 0.6mol 1.8mol 1.2mol
ƽ�� ��a-0.6��mol ��b-1.8��mol 1.2mol
���ԣ���a-0.6��mol=6.3mol×
 ��a=3
��a=3��b-1.8��mol=6.3mol×
 ��b=4.5
��b=4.5�ʴ�Ϊ��3 4.5
��5��I��ƽ��ʱN2Ũ�ȣ�c��N2��=
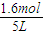
I��ƽ��ʱH2Ũ�ȣ�c��H2��=
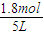
I��ƽ��ʱNH3Ũ�ȣ�c��NH3��=
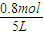
���¶���ƽ�ⳣ��kc=
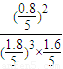 =
=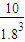 ��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶���ͬƽ�ⳣ����ȣ�
��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶���ͬƽ�ⳣ����ȣ����ƽ��ʱ�����������ΪVL����
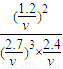 =
=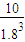 ����V=7.5
����V=7.5�ʴ�Ϊ��7.5
��6������ʼʱ������ѹǿΪp����ʼʱ������ѹǿp�䣬����������״̬����PV=nRT�ã�
p×5=5RT��p��×7.5=6.3RT������p��=0.84p
�ʴ�Ϊ��0.84
���������⿼���֪ʶ��϶࣬���ʡ�ƽ��״̬�ж��ǿ��Ե��ȵ㣬���û�ѧƽ�ⳣ������������״̬�����ǽ����5����6���ʵĹؼ���

��ϰ��ϵ�д�
�����Ŀ
 �ϳɰ���Ӧ�ǡ����������������ķ�Ӧ�����û�кϳɰ���Ӧ������������������ô����ˣ���֪�ϳɰ��ķ�ӦΪN2��g��+3H2��g��?2NH3��g����H=-92.4kJ?mol-1��
�ϳɰ���Ӧ�ǡ����������������ķ�Ӧ�����û�кϳɰ���Ӧ������������������ô����ˣ���֪�ϳɰ��ķ�ӦΪN2��g��+3H2��g��?2NH3��g����H=-92.4kJ?mol-1�� ת����= .
ת����= .