��Ŀ����
����Ŀ��
���᳦��ҩ�����Ч�ɷ�H�ĺϳ�·�����£�

��1��H���������������__________�� Y��������__________��
��2������X��__________�� Y��Z�ķ�Ӧ������__________��
��3��Z�����������__________��ԭ�ӹ�ƽ�档
��4��д��E����������������Һ��Ӧ�Ļ�ѧ����ʽ��_____________________��
��5��ͬʱ��������������E��ͬ���칹����__________�֡�
����E������ͬ�Ĺ����ţ������ϵ�һ����ȡ������ֻ��2�֡�
��6����֪��![]() �ױ��������������������ʱ������һ��ȡ��������ȡ����������ڡ���λ���������������Ȼ�ʱ��ȡ���ڼ�λ���ݴ���RΪԭ�Ϻϳɻ�����
�ױ��������������������ʱ������һ��ȡ��������ȡ����������ڡ���λ���������������Ȼ�ʱ��ȡ���ڼ�λ���ݴ���RΪԭ�Ϻϳɻ�����![]() ����������������ƺϳ�·�ߣ�______________________________________________��
����������������ƺϳ�·�ߣ�______________________________________________��
���𰸡� �Ȼ����ǻ� �ڼ����ӣ���2-�����ӣ� �Ȼ����������ۣ� ȡ����Ӧ 17 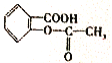 +3NaOH
+3NaOH![]() +CH3COONa+2H2O 4
+CH3COONa+2H2O 4 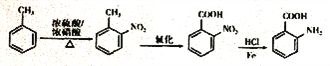
����������������� ˮ������
ˮ������![]() ��
��
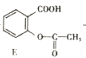 ˮ��ΪF����F�����ǻ������
ˮ��ΪF����F�����ǻ������![]() ���ǻ���������Ũ���ᡢŨ����������������������
���ǻ���������Ũ���ᡢŨ����������������������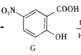 ��
��
��������1�� ���������а������Ȼ����ǻ���
���������а������Ȼ����ǻ��� ![]() ���������ڼ����ӡ�
���������ڼ����ӡ�
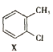
��2��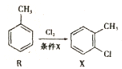 ���������ڱ���������±��ԭ�ӣ�����X�����ۣ�
���������ڱ���������±��ԭ�ӣ�����X�����ۣ� ![]() ����������Ӧ
����������Ӧ![]() �ķ�Ӧ������ȡ����Ӧ��
�ķ�Ӧ������ȡ����Ӧ��
��3��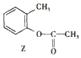 ���ӱ������ʻ�����ƽ��ṹ�������������17��ԭ�ӹ�ƽ�档
���ӱ������ʻ�����ƽ��ṹ�������������17��ԭ�ӹ�ƽ�档
��4�� ����������������Һ����ˮ�ⷴӦ��ѧ����ʽ����
����������������Һ����ˮ�ⷴӦ��ѧ����ʽ����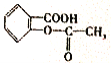 +3NaOH
+3NaOH![]() +CH3COONa+2H2O ��
+CH3COONa+2H2O ��
��5��ͬʱ������������E������ͬ�Ĺ����ţ������ϵ�һ����ȡ������ֻ��2�ֵ�E��ͬ���칹����
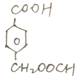
��4�֡�
��6���Լױ�Ϊԭ�Ϻϳɻ�����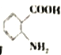 ����Ҫ�������������ٰѼ�����Ϊ�Ȼ�������������ԭΪ�������ϳ�·��Ϊ��
����Ҫ�������������ٰѼ�����Ϊ�Ȼ�������������ԭΪ�������ϳ�·��Ϊ��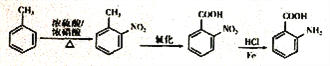 ��
��



