��Ŀ����
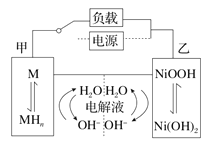
����Ŀ��ij������ȤС����е��ԭ����ʵ��̽�����������µ�ʵ�飺��ͭΪ�缫������ͼ��ʾ��װ�õ�ⱥ��ʳ��ˮ��
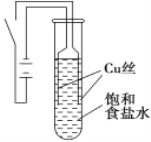
ʵ������ͨ��Դ30 s�ڣ������������ְ�ɫ���ǣ�֮���ɳȻ�ɫ���ǣ���ʱ�ⶨ��Һ��pHԼΪ10��һ��ʱ����Թܵײ��ۼ�������ɫ��������Һ��Ϊ��ɫ��
�������ϣ�
���� | �Ȼ�ͭ | ������ͭ | ��������ͭ | �Ȼ���ͭ |
��ɫ | �������ɫ��Ũ��Һ����ɫ��ϡ��Һ����ɫ | ��ɫ | �Ȼ�ɫ | ��ɫ |
��ͬ�¶���CuCl���ܽ�ȴ���CuOH
����˵���������
A.��Ӧ������������Һ�ʼ���
B.�����Ϸ����ĵ缫��ӦΪ��2H2O + 2e�T H2��+ 2OH
C.��ʵ�鿴����������ͭ���в��ȶ���
D.�Թܵײ���ɫ�Ĺ�����л�ԭ��
���𰸡�A
��������
���ʱ��ͭ��������������ͭʧ����������ͭ���ӽ�����Һ����ͭ���Ӻ������������Ȼ���ͭ��ɫ��������ʱ��Һ�е�����������Ũ�Ⱥ�С�����Խ�������˲���������������ͭ������������ͬ�¶���CuCl���ܽ�ȴ���CuOH�����30s�����˳�����ת�������Ȼ���ͭ��Ϊ��������ͭ������ɫ������ɳȻ�ɫ���ǣ���ʱ�ⶨ��Һ��pHԼΪ10����Һ��ʾ���ԣ�������������ͭ���ȶ������һ��ʱ���ᷢ���ֽ����ɺ�ɫ��������ͭ��
A. ���������Ϣ֪���������з�Ӧ���̣�������2Cu-2e=2Cu+��������2H2O + 2e�T H2��+ 2OH���ܷ�Ӧ��2Cu+2H2O![]() H2��+2CuOH��2CuOH= Cu2O+H2O����˷�Ӧ��������Һ�����ԣ���A����
H2��+2CuOH��2CuOH= Cu2O+H2O����˷�Ӧ��������Һ�����ԣ���A����
B. ���������ǵ������ˮ��������������ӵõ��ӵĻ�ԭ��Ӧ�������ĵ缫��ӦΪ��2H2O + 2e�T H2��+ 2OH����B��ȷ��
C. ��ʵ����������ɫ�ı仯֪��2CuOH= Cu2O+H2O������������ͭ���в��ȶ��ԣ���C��ȷ��
D. �Թܵײ���ɫ�Ĺ�����������ͭ�����е�CuԪ����+1�ۣ����л�ԭ�ԣ���D��ȷ��
��ѡA��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.100 0 mol��L��1��HCl����Һ�ζ������(�ü�����ָʾ��)����ش��������⣺
��1���ζ�ʱ��ʢװ����NaOH��Һ����������Ϊ________��
��2���ζ����յ����ɫ�仯Ϊ____________��
��3������ѧ����ʵ���������������ƽ��ʵ�飬���ݼ�¼���£����ʱ���ı���Һ�����Ϊ__________�����������NaOH��Һ�����ʵ���Ũ��Ϊ______��(������λ��Ч����)
ʵ�� ��� | ����NaOH��Һ�����/mL | 0.100 0 mol��L��1 HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.29 |
2 | 25.00 | 1.00 | 31.00 |
3 | 25.00 | 1.00 | 27.31 |