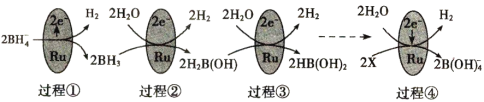
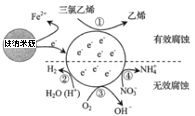
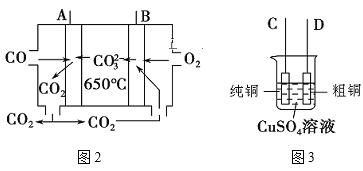
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ2019Ρξ3‘¬21»’ «ΒΎΕΰ °ΤΏΫλΓΑ άΫγΥ°»’Γ±Θ§±ΘΜΛΥ°Ή ‘¥Θ§Κœάμάϊ”ΟΖœΥ°ΫΎ ΓΥ°Ή ‘¥Θ§Φ”«ΩΖœΥ°ΒΡΜΊ ’άϊ”Ο“―±Μ‘Ϋά¥‘ΫΕύΒΡ»ΥΥυΙΊΉΔΓΘ“―÷ΣΘΚΡ≥Έό…ΪΖœΥ°÷–Ω…ΡήΚ§”–H+ΓΔNH4+ΓΔFe3+ΓΔAl3+ΓΔMg2+ΓΔNa+ΓΔNO3-ΓΔCO32-ΓΔSO42-÷–ΒΡΦΗ÷÷Θ§ΈΣΖ÷ΈωΤδ≥…Ζ÷Θ§Ζ÷±π»ΓΖœΥ°―υΤΖ100![]() Θ§Ϋχ––ΝΥ»ΐΉι Β―ιΘ§Τδ≤ΌΉςΚΆ”–ΙΊΆΦœώ»γœ¬ΆΦΥυ ΨΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ§Ϋχ––ΝΥ»ΐΉι Β―ιΘ§Τδ≤ΌΉςΚΆ”–ΙΊΆΦœώ»γœ¬ΆΦΥυ ΨΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ

Θ®1Θ©ΗυΨί…œ ω3Ήι Β―ιΩ…“‘Ζ÷ΈωΖœΥ°÷–“ΜΕ®≤Μ¥φ‘ΎΒΡ“θάκΉ” «_______Θ§“ΜΕ®¥φ‘ΎΒΡ―τάκΉ” «_____ΓΘ
Θ®2Θ©Ζ÷ΈωΆΦœώΘ§‘Ύ‘≠»ή“Κ÷–cΘ®NH4+Θ©”κcΘ®Al3+Θ©ΒΡ±»÷ΒΈΣ_______
Θ®3Θ© »τΆ®Ιΐ Β―ι»ΖΕ®‘≠ΖœΥ°÷–cΘ®Na+Θ©=0.14mol/LΘ§ ‘≈–Εœ‘≠ΖœΥ°÷–NO3- «Ζώ¥φ‘Ύ?_____Θ®ΧνΓΑ¥φ‘ΎΓ±ΓΑ≤Μ¥φ‘ΎΓ±ΜρΓΑ≤Μ»ΖΕ®Γ±Θ©ΓΘ»τ¥φ‘ΎΘ§cΘ®NO3-Θ©=______mol/LΓΘΘ®»τ≤Μ¥φ‘ΎΜρ≤Μ»ΖΕ®‘ρ¥ΥΩ’≤ΜΧνΘ©ΓΘ
ΓΨ¥πΑΗΓΩCO32- Na+ΓΔH+ΓΔAl3+ΓΔNH4+ 1ΘΚ1 ¥φ‘Ύ 0.36
ΓΨΫβΈωΓΩ
”…Έό…ΪΖœΥ°Ω…÷Σ»ή“Κ÷–ΈόFe3+Θ§ΗυΨί Β―ιΔΌΩ…÷Σ»ή“Κ÷–Κ§”–Na+Θ§ΗυΨί Β―ιΔΎΩ…÷Σ»ή“Κ÷–Κ§”–SO42-Θ§ΗυΨί Β―ιΔέΩ…÷Σ»ή“Κ÷–Κ§”–H+ΚΆAl3+Θ§»ή“Κ÷–“ΜΕ®≤ΜΚ§Fe3+ΓΔMg2+Θ§“ρΈΣCO32-”κAl3+≤ΜΡήΙ≤¥φΘ§»ή“Κ÷–ΈόCO32-Θ§‘ρ»ή“Κ÷–¥φ‘ΎΒΡάκΉ”ΈΣNa+ΓΔAl3+ΓΔNH4+ΓΔH+ΓΔSO42-Θ§“ΜΕ®≤Μ¥φ‘ΎΒΡάκΉ””–Fe3+ΓΔMg2+ΓΔCO32-ΓΘ
Θ®1Θ©”…Έό…ΪΖœΥ°Ω…÷Σ»ή“Κ÷–ΈόFe3+Θ§ΗυΨί Β―ιΔΌΩ…÷Σ»ή“Κ÷–Κ§”–Na+Θ§ΗυΨί Β―ιΔΎΩ…÷Σ»ή“Κ÷–Κ§”–SO42-Θ§ΗυΨί Β―ιΔέΩ…÷Σ»ή“Κ÷–Κ§”–H+ΚΆAl3+Θ§»ή“Κ÷–“ΜΕ®≤ΜΚ§Fe3+ΓΔMg2+Θ§“ρΈΣCO32-”κAl3+≤ΜΡήΙ≤¥φΘ§»ή“Κ÷–ΈόCO32-Θ§‘ρ»ή“Κ÷–¥φ‘ΎΒΡάκΉ”ΈΣNa+ΓΔAl3+ΓΔNH4+ΓΔH+ΓΔSO42-Θ§“ΜΕ®≤Μ¥φ‘ΎΒΡάκΉ””–Fe3+ΓΔMg2+ΓΔCO32-Θ§Ι ¥πΑΗΈΣΘΚCO32-ΘΜNa+ΓΔH+ΓΔAl3+ΓΔNH4+ΘΜ
Θ®2Θ©“―÷ΣΝρΥα±Β≥ΝΒμΈΣ2.33gΘ§‘ρnΘ®SO42-Θ©ΈΣ0.01molΘ§ΗυΨίΆΦœσΩ…÷Σ”κAlΘ®OHΘ©3ΒΡOH-ΈΣΒΡΈο÷ ΒΡΝΩ0.007molΘ§”…ΖΫ≥Χ ΫAlΘ®OHΘ©3+OH-=AlO2-+2H2OΩ…÷ΣΫΪ¬ΝάκΉ”≥ΝΒμ–η“Σ«β―θΜ·ΡΤ0.021molΘ§Υυ“‘»ή“Κ÷–H+œϊΚΡ«β―θΜ·ΡΤ0.014molΘ§«βάκΉ”ΒΡΈο÷ ΒΡΝΩ «0.014molΘ§”…ΖΫ≥Χ ΫNH4++OH-=NH3H2OΩ…÷ΣœϊΚΡ«β―θΜ·ΡΤ0.007molΘ§Υυ“‘οßΗυάκΉ”ΒΡΈο÷ ΒΡΝΩ «0.007molΘ§‘ρ‘≠»ή“Κ÷–cΘ®NH4+Θ©”κcΘ®Al3+Θ©ΒΡ±»÷ΒΈΣ1ΘΚ1Θ§Ι ¥πΑΗΈΣΘΚ1:1ΘΜ
Θ®3Θ©”…Χβ“βΩ…÷ΣΘ§¬ΝάκΉ”ΈΣ0.007molΓΔ«βάκΉ”ΈΣ0.014molΓΔοßΗυάκΉ”ΈΣ0.007molΘ§ΝρΥαΗυΒΡΈο÷ ΒΡΝΩΈΣ0.01molΘ§”…ΒγΚ… ΊΚψΩ…÷ΣΘ§»ή“Κ÷–±Ί»Μ”–œθΥαΗυάκΉ”Θ§œθΥαΗυΈο÷ ΒΡΝΩΈΣΘ®0.007molΓΝ3+0.014mol+0.007molΘ©ΓΣ0.01molΓΝ2=0.036molΘ§‘ρcΘ®NO3-Θ©=0.36mol/LΘ§Ι ¥πΑΗΈΣΘΚ0.36ΓΘ

 ΩΣ–ΡΝΖœΑΩΈΩΈΝΖ”κΒΞ‘ΣΦλ≤βœΒΝ–¥πΑΗ
ΩΣ–ΡΝΖœΑΩΈΩΈΝΖ”κΒΞ‘ΣΦλ≤βœΒΝ–¥πΑΗ ΩΣ–Ρ ‘ΨμΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ
ΩΣ–Ρ ‘ΨμΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗΓΨΧβΡΩΓΩ≤ίΥανή”ΟΆΨΙψΖΚΘ§Ω…”Ο”Ύ÷Η ΨΦΝΚΆ¥ΏΜ·ΦΝΒΡ÷Τ±ΗΓΘ”ΟΚ§νήΖœΝœΘ®÷ς“Σ≥…Ζ÷ΈΣ![]() Θ§ΜΙΚ§”–“ΜΕ®ΝΩΒΡ
Θ§ΜΙΚ§”–“ΜΕ®ΝΩΒΡ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() Β»Θ©÷Τ±Η≤ίΥανήΨßΧε
Β»Θ©÷Τ±Η≤ίΥανήΨßΧε![]() ΒΡΙΛ“ΒΝς≥Χ»γœ¬ΆΦΥυ ΨΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΒΡΙΛ“ΒΝς≥Χ»γœ¬ΆΦΥυ ΨΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
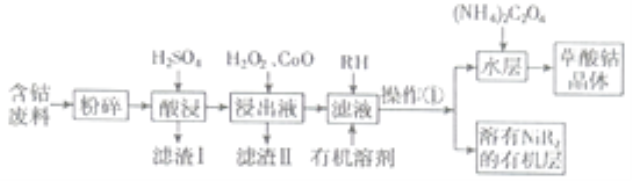
“―÷ΣΘΚΔΌ≤ίΥανήΨßΧεΡ―»ή”ΎΥ°
ΔΎ![]() ΈΣ”–ΜζΈοΘ®Ρ―ΒγάκΘ©
ΈΣ”–ΜζΈοΘ®Ρ―ΒγάκΘ©
ΔέœύΙΊΫπ τάκΉ”![]() –Έ≥…«β―θΜ·Έο≥ΝΒμΒΡ
–Έ≥…«β―θΜ·Έο≥ΝΒμΒΡ![]() ΖΕΈß»γœ¬ΘΚ
ΖΕΈß»γœ¬ΘΚ
Ϋπ τάκΉ” |
|
|
|
|
ΩΣ Φ≥ΝΒμΒΡ | 7.5 | 2.7 | 3.4 | 6.9 |
≥ΝΒμΆξ»ΪΒΡ | 9.0 | 3.7 | 4.7 | 8.9 |
Θ®1Θ©¬Υ‘ϋΔώΒΡ≥…Ζ÷_______ΓΘ
Θ®2Θ©![]() «“Μ÷÷¬Χ…Ϊ―θΜ·ΦΝΘ§–¥≥ωΦ”»κ
«“Μ÷÷¬Χ…Ϊ―θΜ·ΦΝΘ§–¥≥ωΦ”»κ![]() Κσ»ή“Κ÷–ΖΔ…ζΒΡ÷ς“ΣΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ______ΓΘ
Κσ»ή“Κ÷–ΖΔ…ζΒΡ÷ς“ΣΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ______ΓΘ
Θ®3Θ©Φ”»κ―θΜ·νήΒςΫΎΫΰ≥ω“ΚΒΡ![]() ΒΡΖΕΈß «______ΓΘ
ΒΡΖΕΈß «______ΓΘ
Θ®4Θ©Φ”»κ”–Μζ»ήΦΝΒΡΡΩΒΡ «______ΓΘ
Θ®5Θ©»τΫω¥”≥ΝΒμΉΣΜ·Ϋ«Ε»ΩΦ¬«Θ§ΡήΖώάϊ”ΟΖ¥”Π![]() ΫΪ
ΫΪ![]() ΉΣΜ·ΈΣ
ΉΣΜ·ΈΣ![]() ____Θ®ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±Θ©Θ§ΥΒΟςάμ”…ΘΚ______ΓΘΘ®“―÷Σ
____Θ®ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±Θ©Θ§ΥΒΟςάμ”…ΘΚ______ΓΘΘ®“―÷Σ![]() Θ§
Θ§![]() Θ©
Θ©
Θ®6Θ©ΈΣ≤βΕ®÷ΤΒΟΒΡ≤ίΥανήΨßΧε―υΤΖΒΡ¥ΩΕ»Θ§œ÷≥Τ»Γ―υΤΖ![]() Θ§œ»”Ο Β± ‘ΦΝΫΪΤδΉΣΜ·Θ§œΓ ΆΚσΒΟΒΫ¥ΩΨΜΒΡ≤ίΥαοß»ή“Κ
Θ§œ»”Ο Β± ‘ΦΝΫΪΤδΉΣΜ·Θ§œΓ ΆΚσΒΟΒΫ¥ΩΨΜΒΡ≤ίΥαοß»ή“Κ![]() ΓΘ“Τ»Γ
ΓΘ“Τ»Γ![]() ΗΟ»ή“ΚΦ”»κΙΐΝΩΒΡœΓΝρΥαΥαΜ·Θ§”Ο
ΗΟ»ή“ΚΦ”»κΙΐΝΩΒΡœΓΝρΥαΥαΜ·Θ§”Ο![]() ΗΏΟΧΥαΦΊ»ή“ΚΒΈΕ®Θ§Β±»ή“Κ”…__________Θ®Χν―’…Ϊ±δΜ·Θ©Θ§œϊΚΡΗΏΟΧΥαΦΊ»ή“Κ
ΗΏΟΧΥαΦΊ»ή“ΚΒΈΕ®Θ§Β±»ή“Κ”…__________Θ®Χν―’…Ϊ±δΜ·Θ©Θ§œϊΚΡΗΏΟΧΥαΦΊ»ή“Κ![]() Θ§ΦΤΥψ≤ίΥανήΨßΧε―υΤΖΒΡ¥ΩΕ»ΈΣ__________
Θ§ΦΤΥψ≤ίΥανήΨßΧε―υΤΖΒΡ¥ΩΕ»ΈΣ__________![]() ΓΘΘ®”ΟΚ§
ΓΘΘ®”ΟΚ§![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΒΡ¥ζ ΐ Ϋ±μ ΨΘ©Θ®“―÷Σ
ΒΡ¥ζ ΐ Ϋ±μ ΨΘ©Θ®“―÷Σ![]() ΒΡΡΠΕϊ÷ ΝΩΈΣ
ΒΡΡΠΕϊ÷ ΝΩΈΣ![]() Θ©
Θ©
ΓΨΧβΡΩΓΩ―ΈΥαΚΆ«β―θΜ·ΡΤ «ΙΛ“Β…œ÷Ί“ΣΒΡΜ·ΙΛ‘≠ΝœΘ§“≤ « Β―ι “άο≥ΘΦϊΒΡΜ·―ß ‘ΦΝΓΘ”ϊ≤βΕ®Ρ≥NaOH»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»Θ§Ω…”Ο0.1000 molΓΛL-1 HCl±ξΉΦ»ή“ΚΫχ––÷–ΚΆΒΈΕ®(”ΟΖ”ΧΣΉς÷Η ΨΦΝ)ΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΒΈΕ® ±Θ§ ΔΉΑ¥ΐ≤βNaOH»ή“ΚΒΡ“«ΤςΟϊ≥ΤΈΣ_____ΓΘ
Θ®2Θ©Φν ΫΒΈΕ®Ιή”Ο’τΝσΥ°œ¥ΨΜΚσΘ§Ϋ”œ¬ά¥”ΠΗΟΫχ––ΒΡ≤ΌΉς «_________ΓΘ
Θ®3Θ©»τΦΉ―ß…ζ‘Ύ Β―ιΙΐ≥Χ÷–Θ§Φ«¬ΦΒΈΕ®«ΑΒΈΕ®ΙήΡΎ“ΚΟφΕΝ ΐΈΣ0.50 mLΘ§ΒΈΕ®Κσ“ΚΟφ»γΆΦΘ§‘ρ¥Υ ±œϊΚΡ±ξΉΦ»ή“ΚΒΡΧεΜΐΈΣ_____ΓΘ
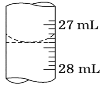
““―ß…ζΉωΝΥ»ΐΉιΤΫ–– Β―ιΘ§ ΐΨίΦ«¬Φ»γœ¬ΘΚ
Β―ι–ρΚ≈ | ¥ΐ≤βNaOH»ή“ΚΒΡΧεΜΐ/mL | 0.1000molΓΛL-1HCl»ή“ΚΒΡΧεΜΐ/mL | |
ΒΈΕ®«ΑΩΧΕ» | ΒΈΕ®ΚσΩΧΕ» | ||
1 | 25.00 | 0.11 | 25.10 |
2 | 25.00 | 1.56 | 33.30 |
3 | 25.00 | 0.21 | 25.22 |
Θ®4Θ©―ûÅœ ωΚœάμ ΐΨίΘ§ΦΤΥψ≥ω¥ΐ≤βNaOH»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ______(±ΘΝτΥΡΈΜ”––ß ΐΉ÷)ΓΘ
Θ®5Θ©œ¬Ν–ΡΡ–©≤ΌΉςΜα Ι≤βΕ®ΫαΙϊΤΪΗΏ_____ (Χν–ρΚ≈)ΓΘ
AΘ°ΉΕ–ΈΤΩ”Ο’τΝσΥ°œ¥ΨΜΚσ‘Ό”Ο¥ΐ≤β“Κ»σœ¥
BΘ°Υα ΫΒΈΕ®Ιή”Ο’τΝσΥ°œ¥ΨΜΚσ‘Ό”Ο±ξΉΦ“Κ»σœ¥
CΘ°ΒΈΕ®«ΑΥα ΫΒΈΕ®ΙήΦβΕΥΤχ≈ίΈ¥≈≈≥ΐΘ§ΒΈΕ®ΚσΤχ≈ίœϊ ß
DΘ°ΒΈΕ®«ΑΕΝ ΐ’ΐ»ΖΘ§ΒΈΕ®ΚσΗ© ”ΒΈΕ®ΙήΕΝ ΐ
Θ®6Θ©ΒΈΕ®¥οΒΫ÷’ΒψΒΡ±ξ÷Ψ «________ΓΘ