��Ŀ����
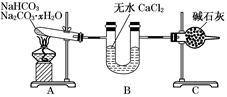
(9��)����NaHCO3��Na2CO3��xH2O�Ļ���Ϊ�˲ⶨxֵ��ijͬѧ������ͼ��ʾ��װ�ý���ʵ��(CaCl2����ʯ�Ҿ�����)��
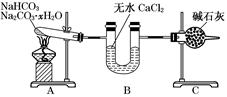
(1)Aװ�õ�������_________________________________________________________��
(2)Bװ�õ�������_________________________________________________________��
(3)Cװ�õ�������_________________________________________________________��
(4)����װ��A���Թ���װ��NaHCO3��Na2CO3��xH2O�Ļ����3.7 g���þƾ��Ƽ��ȵ���Ӧ��ȫ����ʱB������1.89 g��C������0.22 g����x��ֵΪ______��
(5)��װ�û����Ǻ����ƣ�����ʹ�ⶨ���ƫС��Ӧ��θĽ�________��Ϊʲô��________________________________________________________________________
________________________________________________________________________��
(1)���ȣ�ʹNaHCO3�ֽ⣬ʹNa2CO3��xH2Oʧˮ (2��)
(2)���շ�Ӧ�����ɵ�ˮ��(1��) (3)���շ�Ӧ�����ɵ�CO2(2��)
(4)10 (2��)
(5)��Cװ�ú��ټ�һ��װ�м�ʯ�ҵ�U�ιܡ�Ϊ�˷�ֹ�����е�CO2��H2O��Cװ���еļ�ʯ������(2��)
��������
��������������ʵ��Ŀ���Dzⶨ��NaHCO3��Na2CO3��xH2O�Ļ�����е�xֵ������ʵ��װ�÷����ó���Aװ��Ϊ����װ�ã�������Ӧ��2NaHCO3 Na2CO3+CO2��+H2O��Na2CO3��xH2O=
Na2CO3+ xH2O��Bװ��Ϊ����װ�ã�������Ϊ����ˮ�֣�Cװ��Ϊװ�м�ʯ�ҵĸ���ܣ�Ҳ��������ã�����ͬʱҲ������CO2�����á���4��B������1.89 gΪH2O��������C������0.22 gΪCO2���������ɻ�ѧ����ʽ�ɵ����¹�ϵʽ��
Na2CO3+CO2��+H2O��Na2CO3��xH2O=
Na2CO3+ xH2O��Bװ��Ϊ����װ�ã�������Ϊ����ˮ�֣�Cװ��Ϊװ�м�ʯ�ҵĸ���ܣ�Ҳ��������ã�����ͬʱҲ������CO2�����á���4��B������1.89 gΪH2O��������C������0.22 gΪCO2���������ɻ�ѧ����ʽ�ɵ����¹�ϵʽ��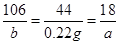 ��
��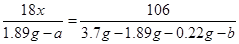 �����
�����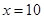 ����5����װ��������Cװ�ü�ʯ��Ҳ�����տ����е�CO2��ˮ���ᵼ�����ս��ƫС����˿���Cװ�ú�������һ��װ�м�ʯ�ҵĸ���ܻ�U�ܡ�
����5����װ��������Cװ�ü�ʯ��Ҳ�����տ����е�CO2��ˮ���ᵼ�����ս��ƫС����˿���Cװ�ú�������һ��װ�м�ʯ�ҵĸ���ܻ�U�ܡ�
���㣺NaHCO3��Na2CO3������
�����������Ƕ�NaHCO3��Na2CO3���ʵĿ��飬����ʵ���⣬Ӧ���������ȷʵ��Ŀ�ģ���ζ�����ʵ��װ�õ����ͣ�Ӧ��ȷÿ��ʵ��װ�õ����ã��ٴΣ����������װ���Լ��������̣��ó�ʵ��ԭ�������Ϸ����Լ�������н��

(9��) ����A��B��C��D��E���ֿ�����ǿ����ʣ�������ˮ�пɵ��������������(�������Ӳ��ظ�)��
|
������ |
H+��Na+��A13+��Ag+��Ba2+ |
|
������ |
OH����C1����CO32����NO3����SO4�� |
��֪����A��B����Һ�ʼ��ԣ�C��D��E��Һ�����ԣ�
��A��Һ��E��Һ��Ӧ�����������г���������A��Һ��C��Һ��Ӧֻ�����������
��D��Һ������������Һ��Ӧ���ܲ���������Cֻ����D��Ӧ����������
������������������������
�Իش��������⣺
(1)д��A��Һ��E��Һ��Ӧ�Ļ�ѧ����ʽ�� _________��
(2)д��E��Һ�������B��Һ��Ӧ�����ӷ���ʽ�� ____��
(3)��֪��NaOH(aq)+HNO3(aq)=NaNO3(aq)+H2O(1) ��H=��akJ��mol��1����д��B��C��ϡ��Һ��Ӧ���Ȼ�ѧ����ʽ�� _____________________��
 _______��________
_______��________
 ��
��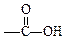 ��
��