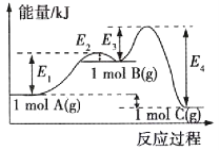
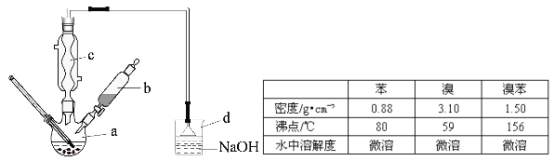
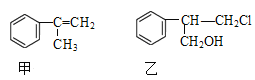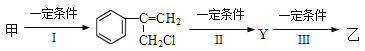��Ŀ����
����Ŀ������������Ԫ�� X��Y��Z��W ԭ�������������� X��Z��W �����γ����������X��һ���⻯����ӿռ乹��Ϊ�����ͣ�YW���ȼҵ����Ҫԭ�ϣ�Z������������Ϊ4����ش��������⣺
(1)��ʾ�ȼҵ����ԭ���Ļ�ѧ����ʽΪ____________________________________��
(2)X����һ���⻯��X2H4����Ϊ����ƽ���ȼ�ϣ���ṹʽΪ__________________ ��
(3)Y���������У���һ�ּȺ����Ӽ��ֺ����ۼ�����������ĵ���ʽΪ ___________��
(4)Z�����������ھ��壬��ҵ�Ʊ�Z���ʵĻ�ѧ����ʽΪ________________________��
(5)W�����Ƕ��Ժܴ����Ϣ�����塣��ҵ����X��̬�⻯���Ũ��Һ����W�����Ƿ�й¶��д����Ӧ�Ļ�ѧ����ʽ_________________________________________ ��
���𰸡�2NaCl+2H2O![]() 2NaOH+H2��+Cl2��
2NaOH+H2��+Cl2�� ![]()
![]() 2C+SiO2
2C+SiO2![]() 2CO��+Si 8NH3+3Cl2=6NH4Cl+N2
2CO��+Si 8NH3+3Cl2=6NH4Cl+N2
��������
����������Ԫ�� X��Y��Z��W ԭ��������������X��Z��W �����γ����������X��һ���⻯����ӿռ乹��Ϊ�����ͣ����⻯��Ϊ��������XΪNԪ�أ�YW���ȼҵ����Ҫԭ�ϣ�������ΪNaCl����YΪNa��WΪClԪ�أ�Z������������Ϊ4��ԭ����������Na����ZΪSiԪ�أ��ݴ˽��н��
���ݷ�����֪��XΪNԪ�أ�YΪNa��ZΪSi��WΪClԪ�ء�
(1)�ȼҵ�е�ⱥ��ʳ��ˮ�����������ơ��������������÷�Ӧ�Ļ�ѧ����ʽΪ��2NaCl+2H2O![]() 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����
(2)X2H4ΪN2H4����ṹ��ʽΪNH2-NH2��ÿ����ԭ���γ�������ѧ����N2H4�ĽṹʽΪ![]() ��
��
(3)Na���������мȺ����Ӽ��ֺ����ۼ���Ϊ�������ƣ���������Ϊ���ӻ���������ʽΪ![]() ��
��
(4)ZΪSiԪ�أ���������ΪSiO2��������������ԭ�Ӿ��壻��ҵ����̼����������ڸ����·�Ӧ��ȡ�裬�÷�Ӧ�Ļ�ѧ����ʽΪ��2C+SiO2![]() 2CO��+Si��
2CO��+Si��
(5)W����Ϊ�����������Ƕ��Ժܴ����Ϣ�����壬X��̬�⻯��Ϊ�����������백����Ӧ�����Ȼ�狀͵�������ϵ����غ㡢�����غ���ƽ�÷�Ӧ�Ļ�ѧ����ʽΪ��8NH3+3Cl2=6NH4Cl+N2��

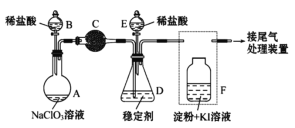
����Ŀ����ͼ������������̽��SO2�����ʡ�ʵ��ʱ��Na2SO3�����ϵμ���Ũ���ᣬ��������һ������������档
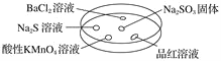
�±��ж�ʵ������������������Ľ��Ͳ���ȷ����(����)
ѡ�� | ʵ������ | ���� |
A | BaCl2��Һ����� | SO2��BaCl2��Һ��Ӧ������BaSO3���� |
B | Na2S��Һ����� | SO2��Na2S��Һ��Ӧ������S���� |
C | ����KMnO4��Һ��ɫ | SO2���л�ԭ�� |
D | Ʒ����Һ��ɫ | SO2����Ư���� |
A. A B. B C. C D. D