��Ŀ����
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������100 mL 1 mol���̣�1��ϡ���ᡣ

�ɹ�ѡ�õ������У�
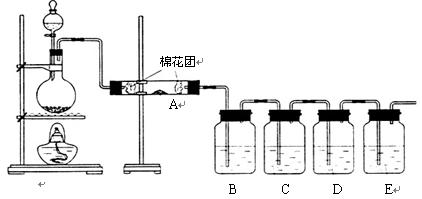
�ٽ�ͷ�ιܣ�����ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
(1)ʢ��Ũ������Լ�ƿ��ǩ��Ӧӡ�����о�ʾ����е�

(2)����ϡ����ʱ����ȱ�ٵ������� (д��������)��
(3)�����㣬����100 mL1 mol���̣�1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ mL����ȡ����ʱӦѡ�� mL������Ͳ��
A��10 mL B��50 mL C��100 mL D��200mL
(4)���ձ���ϡ��Ũ�����ʵ�����Ϊ
������ϡ�����У�����Ũ�����մ�����ϣ���������Ϊ
 ��
��
(5)�������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1 mol���̣�1�����ƹ��������и�������������������ԭ�� ��
A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ����
B������ƿ������ˮϴ�Ӻ�δ���������������ˮ
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D��ת����Һʱ��������������Һ��������ƿ����
E������ʱ����������ƿ�̶��߽��ж���
F�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�

�ɹ�ѡ�õ������У�
�ٽ�ͷ�ιܣ�����ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
(1)ʢ��Ũ������Լ�ƿ��ǩ��Ӧӡ�����о�ʾ����е�

(2)����ϡ����ʱ����ȱ�ٵ������� (д��������)��
(3)�����㣬����100 mL1 mol���̣�1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ mL����ȡ����ʱӦѡ�� mL������Ͳ��
A��10 mL B��50 mL C��100 mL D��200mL
(4)���ձ���ϡ��Ũ�����ʵ�����Ϊ
������ϡ�����У�����Ũ�����մ�����ϣ���������Ϊ
 ��
��(5)�������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1 mol���̣�1�����ƹ��������и�������������������ԭ�� ��
A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ����
B������ƿ������ˮϴ�Ӻ�δ���������������ˮ
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D��ת����Һʱ��������������Һ��������ƿ����
E������ʱ����������ƿ�̶��߽��ж���
F�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�
(1)D(1��) (2)��������100mL������ƿ(��1��) (3)5.4(2��) A(1��)
(4)��Ũ�������ձ��ڻ���ע��װ��ˮ���ձ��У����ò��������Ͻ��裬ʹ��������Ѹ��ɢȥ(1��) �����ô���ˮ��ϴ������Ϳ��NaHC O3��ϡ��Һ(1��) (5)ACE(2��)
O3��ϡ��Һ(1��) (5)ACE(2��)
(4)��Ũ�������ձ��ڻ���ע��װ��ˮ���ձ��У����ò��������Ͻ��裬ʹ��������Ѹ��ɢȥ(1��) �����ô���ˮ��ϴ������Ϳ��NaHC
 O3��ϡ��Һ(1��) (5)ACE(2��)
O3��ϡ��Һ(1��) (5)ACE(2��)��

��ϰ��ϵ�д�
 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�����Ŀ
 ����100mL
����100mL