��Ŀ����
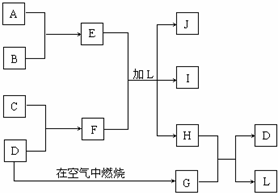 ��ͼÿһ�����е���ĸ����һ�ַ�Ӧ��������
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ������������J�Ǻ�A����Ԫ�صĽ�״��ɫ������IΪNaCl��Һ��D�ǵ���ɫ���嵥�ʣ�����д���пհף�
��1��L�Ļ�ѧʽΪ��
H2O
H2O
����2��F�Ļ�ѧʽΪ��
Na2S
Na2S
����3��д��J���ȷֽⷴӦ�Ļ�ѧ����ʽ��
2Al��OH��3
Al2O3+3H2O
| ||
2Al��OH��3
Al2O3+3H2O
��
| ||
��4��H��G֮�䷴Ӧ�Ļ�ѧ����ʽΪ��
2H2S+SO2�T3S+2H2O
2H2S+SO2�T3S+2H2O
���������ӡ�D�ǵ���ɫ���嵥�ʡ��ɳ����϶�����G�Ƕ������ӡ�J�Ǻ�A����Ԫ�صĽ�״��ɫ�������ɳ����϶�A������J�������������ӡ�IΪNaCl��Һ�����Ƴ�CΪ�ƣ�FΪ���ƣ�BΪCl2��EΪAlCl3��LΪH2O��HΪH2S��H2S��SO2�䷢��������ԭ��Ӧ����S��H2O��֤�������ƶϵ���ȷ�ԣ��������ʵ����ʺ������غ㶨����д��ѧ����ʽ��
����⣺��1����ѧ��ѧϰ���ĵ���ɫ���嵥��ΪS����G�Ƕ���������SO2��Ӧ����S������ΪH2S�����߷�Ӧ����S��ˮ���ʴ�Ϊ��H2O��
��2���ӡ�J�Ǻ�A����Ԫ�صĽ�״��ɫ�������ɳ����϶�A������J�������������ӡ�IΪNaCl��Һ�����Ƴ�CΪ�ƣ�FΪ���ƣ�BΪCl2��EΪAlCl3��LΪH2O��HΪH2S���ʴ�Ϊ��Na2S��
��3�����������ڸ����·ֽ�����Al2O3��H2O�����������غ㶨�ɿ�д����ѧ����ʽ��2Al��OH��3
Al2O3+3H2O��
�ʴ�Ϊ��2Al��OH��3
Al2O3+3H2O��
��4��SO2���������ԣ�H2S��ԭ�ԣ����߷���������ԭ��Ӧ����S��H2O���ʴ�Ϊ��2H2S+SO2�T3S+2H2O��
��2���ӡ�J�Ǻ�A����Ԫ�صĽ�״��ɫ�������ɳ����϶�A������J�������������ӡ�IΪNaCl��Һ�����Ƴ�CΪ�ƣ�FΪ���ƣ�BΪCl2��EΪAlCl3��LΪH2O��HΪH2S���ʴ�Ϊ��Na2S��
��3�����������ڸ����·ֽ�����Al2O3��H2O�����������غ㶨�ɿ�д����ѧ����ʽ��2Al��OH��3
| ||
�ʴ�Ϊ��2Al��OH��3
| ||
��4��SO2���������ԣ�H2S��ԭ�ԣ����߷���������ԭ��Ӧ����S��H2O���ʴ�Ϊ��2H2S+SO2�T3S+2H2O��
������������Ŀ����ѧ����Ԫ�ػ�����֪ʶ����Ϥ�̶ȣ�ͬʱ�ܺõĿ���ѧ��������������������˼ά����������ʱ�õ�����Ϣ����һ���ǰ����е�˳����У�����Ҫ���ڹ��ɣ�����˼ά��ͨ�̿��ǣ�Ѱ��ͻ�ƿڣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 NH4++NH2-
NH4++NH2-
