��Ŀ����
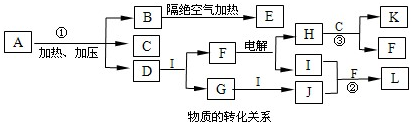
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ��������
����A��B��Ӧ��������E��F��G������C��D��Ӧ��������I��ij�¶��¸÷�Ӧ��ʼ��ijʱ�̵ķ�Ӧ�������������ϱ�����ʾ������д���пհף�
��1������H�ķ���ʽ��
��2����Ӧ�ٵĻ�ѧ����ʽ��
��3����Ӧ�ڵĻ�ѧ����ʽ����ע����Ӧ��������

����A��B��Ӧ��������E��F��G������C��D��Ӧ��������I��ij�¶��¸÷�Ӧ��ʼ��ijʱ�̵ķ�Ӧ�������������ϱ�����ʾ������д���пհף�
��1������H�ķ���ʽ��
HCl
HCl
����2����Ӧ�ٵĻ�ѧ����ʽ��
Cl2+2NaOH=NaClO+NaCl+H2O
Cl2+2NaOH=NaClO+NaCl+H2O
����3����Ӧ�ڵĻ�ѧ����ʽ����ע����Ӧ��������
N2+3H2
2NH3
| ||
| ���¸�ѹ |
N2+3H2
2NH3
��
| ||
| ���¸�ѹ |
��������ⱥ��ʳ��ˮ����Cl2��H2��NaOH������Cl2��NaOH��Ӧ����NaClO��NaCl��H2O�������ʣ���Cl2��H2��Ӧֻ����HCl���ɿ�ͼ��֪AΪNaOH��BΪCl2��CΪH2���ɱ������ݿ�֪n��H2����n��D����n��I��=3��1��2���������غ㶨�ɿ�֪��DΪ˫ԭ�ӷ��ӣ�1molI�к���3molH��1molD��IӦΪNH3����DΪN2���Դ˽��н��
����⣺��ⱥ��ʳ��ˮ����Cl2��H2��NaOH������Cl2��NaOH��Ӧ����NaClO��NaCl��H2O�������ʣ���Cl2��H2��Ӧֻ����HCl���ɿ�ͼ��֪AΪNaOH��BΪCl2��CΪH2���ɱ������ݿ�֪n��H2����n��D����n��I��=3��1��2���������غ㶨�ɿ�֪��DΪ˫ԭ�ӷ��ӣ�1molI�к���3molH��1molD��IӦΪNH3����DΪN2����
��1��BΪCl2��CΪH2�����߷�Ӧ����HCl���ʴ�Ϊ��HCl��
��2��Cl2��NaOH��Ӧ����NaClO��NaCl��H2O�������ʣ���Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaClO+NaCl+H2O��
�ʴ�Ϊ��Cl2+2NaOH=NaClO+NaCl+H2O��
��3����Ӧ��ΪN2��H2�ڴ��������µķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪN2+3H2
2NH3��
�ʴ�Ϊ��N2+3H2
2NH3��
��1��BΪCl2��CΪH2�����߷�Ӧ����HCl���ʴ�Ϊ��HCl��
��2��Cl2��NaOH��Ӧ����NaClO��NaCl��H2O�������ʣ���Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaClO+NaCl+H2O��
�ʴ�Ϊ��Cl2+2NaOH=NaClO+NaCl+H2O��
��3����Ӧ��ΪN2��H2�ڴ��������µķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪN2+3H2
| ||
| ���¸�ѹ |
�ʴ�Ϊ��N2+3H2
| ||
| ���¸�ѹ |
���������⿼��������ƶϣ���Ŀ�ѶȲ���ע��Cl2��NaOH�ķ�Ӧ������������������ж��ǽ�����Ĺؼ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 NH4++NH2-
NH4++NH2- ��ͼÿһ�����е���ĸ����һ�ַ�Ӧ��������
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ��������