��Ŀ����
��1������ͬ�ݻ����ܷ�����A��B�������£�A�г���a g A���壬B�г���a g CH4���壬A��B�ڵ�ѹǿ֮����4��11����A��Ħ������Ϊ ����2��ij�Ȼ�þ��Һ���ܶ�Ϊd g/cm3������þ���ӵ���������Ϊw��a mL����Һ��Cl-�����ʵ���Ϊ ��
��3����״����VL�����ܽ���1Lˮ�У�ˮ���ܶȽ���Ϊ1g/mL����������Һ���ܶ�Ϊ�� g/mL�������Һ�����ʵ����ʵ���Ũ��Ϊ ��
��4���� 11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4 �У�ÿ 1mol CuSO4�������������ʵ����� ��
���𰸡���������1������n=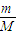 ������������ʵ�����������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȼ���A��Ħ��������
������������ʵ�����������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȼ���A��Ħ��������
��2��������Һ���ܶȺ�þ���ӵ�������������þ���ӵ����ʵ��������ݻ�ѧʽ����Cl-�����ʵ�����
��3������������������ʵ����ʵ�����������Һ���������ܶȼ�����Һ������������������Һ�����ʵ���Ũ�ȣ�
��4�����ݻ��ϼ۵ı仯��ϻ�ѧ����ʽ���㣮
����⣺��1��������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȣ�
��A��Ħ������ΪM��
����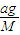 ��
��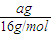 =4��11��
=4��11��
��֮��M=44g/mol��
�ʴ�Ϊ��44g/mol��
��2��aml��Һ������Ϊ��aml×d g/ml=adg��
þ���ӵ�����Ϊ��adg×w=adwg��
þ���ӵ����ʵ���Ϊ��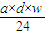 mol��
mol��
��a mL����Һ��Cl-�����ʵ���Ϊ��n��Cl-��=2n��Mg2+��=2×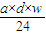 mol=
mol=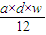 mol��
mol��
�ʴ�Ϊ��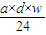 mol��
mol��
��3����״����VL���������ʵ���Ϊ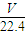 mol������Ϊ
mol������Ϊ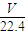 mol×17g/mol=
mol×17g/mol=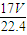 g��
g��
�ܽ���1Lˮ�У�������Һ������Ϊ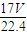 g+1000g�����Ϊ��V=
g+1000g�������V=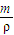 =
=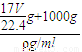 =
=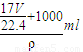 ����
����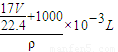 ��
��
����Һ�����ʵ����ʵ���Ũ��Ϊ��c=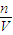 =
=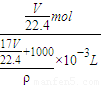 =
=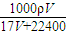 mol/L��
mol/L��
�ʴ�Ϊ��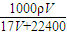 mol/L��
mol/L��
��4���� 11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4�У�P��H3PO4��P�Ļ��ϼ���0������Ϊ+5�ۣ�CuSO4��Cu3P��Cu�Ļ��ϼ���+2�۽���Ϊ+1�ۣ�������ÿ 1molCuSO4�������������ʵ�����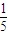 mol��
mol��
�ʴ�Ϊ�� mol��
mol��
���������⿼�����ʵ�������ؼ��㣬��Ŀ�Ѷ��еȣ�ע���йؼ��㹫ʽ�����ã�
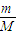 ������������ʵ�����������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȼ���A��Ħ��������
������������ʵ�����������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȼ���A��Ħ����������2��������Һ���ܶȺ�þ���ӵ�������������þ���ӵ����ʵ��������ݻ�ѧʽ����Cl-�����ʵ�����
��3������������������ʵ����ʵ�����������Һ���������ܶȼ�����Һ������������������Һ�����ʵ���Ũ�ȣ�
��4�����ݻ��ϼ۵ı仯��ϻ�ѧ����ʽ���㣮
����⣺��1��������ͬ�¶Ⱥ�����£������ѹǿ֮�ȵ������ʵ���֮�ȣ�
��A��Ħ������ΪM��
����
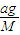 ��
��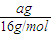 =4��11��
=4��11����֮��M=44g/mol��
�ʴ�Ϊ��44g/mol��
��2��aml��Һ������Ϊ��aml×d g/ml=adg��
þ���ӵ�����Ϊ��adg×w=adwg��
þ���ӵ����ʵ���Ϊ��
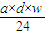 mol��
mol����a mL����Һ��Cl-�����ʵ���Ϊ��n��Cl-��=2n��Mg2+��=2×
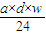 mol=
mol=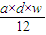 mol��
mol���ʴ�Ϊ��
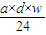 mol��
mol����3����״����VL���������ʵ���Ϊ
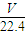 mol������Ϊ
mol������Ϊ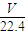 mol×17g/mol=
mol×17g/mol=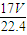 g��
g���ܽ���1Lˮ�У�������Һ������Ϊ
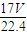 g+1000g�������V=
g+1000g�������V=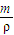 =
=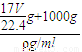 =
=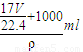 ����
����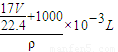 ��
������Һ�����ʵ����ʵ���Ũ��Ϊ��c=
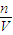 =
=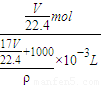 =
=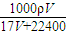 mol/L��
mol/L���ʴ�Ϊ��
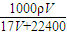 mol/L��
mol/L����4���� 11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4�У�P��H3PO4��P�Ļ��ϼ���0������Ϊ+5�ۣ�CuSO4��Cu3P��Cu�Ļ��ϼ���+2�۽���Ϊ+1�ۣ�������ÿ 1molCuSO4�������������ʵ�����
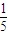 mol��
mol���ʴ�Ϊ��
 mol��
mol�����������⿼�����ʵ�������ؼ��㣬��Ŀ�Ѷ��еȣ�ע���йؼ��㹫ʽ�����ã�

��ϰ��ϵ�д�
�����Ŀ