��Ŀ����
�������ȣ�ClO2�����������Ĵ�ɱ����������
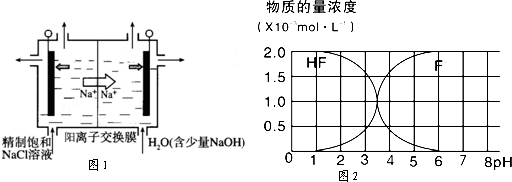
��1����ҵ����NaCl��ԭNaClO3����ClO2�Ĺ�����������ͼ��ʾ��

�ٷ�Ӧ���з�����Ӧ�Ļ�ѧ����ʽΪ��
2NaClO3+2NaCl+2H2SO4=2ClO2��+Cl2��+2Na2SO4+2H2O
�����н�NaClO3��NaCl�����ʵ���֮��1��1.05�Ļ��ˮ��Һ���뷴Ӧ����NaCl�Թ�����Ŀ����______��
�ڷ�Ӧ�������ɵ�ClO2��Cl2��ͨ�������������ClO2�������������Ļ��Һ�������������������ų��ķ�Һ�ɷ���Ҫ��______���ѧʽ����ͬ����
����������������ѭ����ѭ�����õ�������______��
��Ϊ���ClO2�IJ����������������ƣ�NaClO2���뷴Ӧ����Cl2��Ӧ����ClO2���÷�Ӧ�Ļ�ѧ����ʽΪ______��β���е�Cl2����SO2ˮ��Һ���գ��÷�Ӧ�Ļ�ѧ����ʽΪ______��
��2���������ӽ���Ĥ�ָ��ĵ��ص��450g/L NaClO2��Һ�����������Ҳ���ClO2���������Ҳ���H2��NaOH�������й�˵����ȷ����______������ĸ���ţ���
A�����ʱ��������Ϊ����Դ���������ߡ����������������ߡ���Դ����
B���ڵ������У�Na+�������ƶ���ClO2-�������ƶ�
C���ڵ�������������Χ��pH��������
D���������ӷ���ʽ�ɱ�ʾΪ��2ClO2-+2H2O 2ClO2��+H2��+2OH-��
2ClO2��+H2��+2OH-��
�⣺��1����NaCl�Թ������ɻ�ѧ��Ӧ�е�ת����֪��ʹNaClO3��ַ�Ӧ�����NaClO3�������ʣ��ʴ�Ϊ��ʹNaClO3��ַ�Ӧ�����NaClO3�������ʣ�
�������̿�֪���������ų��ķ�Һ�ɷ���ҪΪ�����ơ��Ȼ��ơ����ᣬ��ѧʽ�ֱ�ΪNa2SO4��NaCl��H2SO4���ʴ�Ϊ��Na2SO4��NaCl��H2SO4��
������������ѭ�����м�Һѭ�����ã���ѭ�����õ�����ΪNaOH����NaOH��NaClO��NaCl����
�ʴ�Ϊ��NaOH����NaOH��NaClO��NaCl����
���������ƣ�NaClO2���뷴Ӧ����Cl2��Ӧ����ClO2���������Ȼ��ƣ��÷�ӦΪ2NaClO2+Cl2=2ClO2+2NaCl��Cl2��SO2��Ӧ������������ᣬg�÷�ӦΪCl2+SO2+2H2O=H2SO4+2HCl��
�ʴ�Ϊ��2NaClO2+Cl2=2ClO2+2NaCl��Cl2+SO2+2H2O=H2SO4+2HCl��
��2�����NaClO2��Һ�����������Ҳ���ClO2���������Ҳ���H2��NaOH��������ClO2-ʧȥ���ӣ������������ӵõ����ӣ�
A�����ʱ������������������������������������ʱ��������Ϊ����Դ���������ߡ����������������ߡ���Դ��������A��ȷ��
B���ڵ������У�Na+�������ƶ���ClO2-�������ƶ�����B����
C���ڵ�����������ClO2-ʧȥ��������ClO2����Χ��pH�������䣬��C��ȷ��
D��������ClO2-ʧȥ���ӣ������������ӵõ����ӣ��ڵ������ӷ���ʽ�ɱ�ʾΪ2ClO2-+2H2O 2ClO2��+H2��+2OH-����D��ȷ��
2ClO2��+H2��+2OH-����D��ȷ��
�ʴ�Ϊ��ACD��
��������1����NaCl�Թ�������ٽ�NaClO3��ַ�Ӧ��
�������̿�֪���������ų��ķ�Һ�ɷ���ҪΪ�����ơ��Ȼ��ơ����
������������ѭ�����м�Һѭ�����ã�
���������ƣ�NaClO2���뷴Ӧ����Cl2��Ӧ����ClO2���������Ȼ��ƣ�Cl2��SO2��Ӧ������������
��2�����NaClO2��Һ�����������Ҳ���ClO2���������Ҳ���H2��NaOH��������ClO2-ʧȥ���ӣ������������ӵõ����ӣ��Դ˷�����
�����������Թ�ҵ����NaCl��ԭNaClO3����ClO2�Ĺ������̿�������ķ��롢�ᴿ����ȷ�����ǽ����Ĺؼ���ע�ⷢ���Ļ�ѧ��Ӧ�������Ŀ�ѶȽϴ�
�������̿�֪���������ų��ķ�Һ�ɷ���ҪΪ�����ơ��Ȼ��ơ����ᣬ��ѧʽ�ֱ�ΪNa2SO4��NaCl��H2SO4���ʴ�Ϊ��Na2SO4��NaCl��H2SO4��
������������ѭ�����м�Һѭ�����ã���ѭ�����õ�����ΪNaOH����NaOH��NaClO��NaCl����
�ʴ�Ϊ��NaOH����NaOH��NaClO��NaCl����
���������ƣ�NaClO2���뷴Ӧ����Cl2��Ӧ����ClO2���������Ȼ��ƣ��÷�ӦΪ2NaClO2+Cl2=2ClO2+2NaCl��Cl2��SO2��Ӧ������������ᣬg�÷�ӦΪCl2+SO2+2H2O=H2SO4+2HCl��
�ʴ�Ϊ��2NaClO2+Cl2=2ClO2+2NaCl��Cl2+SO2+2H2O=H2SO4+2HCl��
��2�����NaClO2��Һ�����������Ҳ���ClO2���������Ҳ���H2��NaOH��������ClO2-ʧȥ���ӣ������������ӵõ����ӣ�
A�����ʱ������������������������������������ʱ��������Ϊ����Դ���������ߡ����������������ߡ���Դ��������A��ȷ��
B���ڵ������У�Na+�������ƶ���ClO2-�������ƶ�����B����
C���ڵ�����������ClO2-ʧȥ��������ClO2����Χ��pH�������䣬��C��ȷ��
D��������ClO2-ʧȥ���ӣ������������ӵõ����ӣ��ڵ������ӷ���ʽ�ɱ�ʾΪ2ClO2-+2H2O
 2ClO2��+H2��+2OH-����D��ȷ��
2ClO2��+H2��+2OH-����D��ȷ���ʴ�Ϊ��ACD��
��������1����NaCl�Թ�������ٽ�NaClO3��ַ�Ӧ��
�������̿�֪���������ų��ķ�Һ�ɷ���ҪΪ�����ơ��Ȼ��ơ����
������������ѭ�����м�Һѭ�����ã�
���������ƣ�NaClO2���뷴Ӧ����Cl2��Ӧ����ClO2���������Ȼ��ƣ�Cl2��SO2��Ӧ������������
��2�����NaClO2��Һ�����������Ҳ���ClO2���������Ҳ���H2��NaOH��������ClO2-ʧȥ���ӣ������������ӵõ����ӣ��Դ˷�����
�����������Թ�ҵ����NaCl��ԭNaClO3����ClO2�Ĺ������̿�������ķ��롢�ᴿ����ȷ�����ǽ����Ĺؼ���ע�ⷢ���Ļ�ѧ��Ӧ�������Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 �������ȣ�ClO2��Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
�������ȣ�ClO2��Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������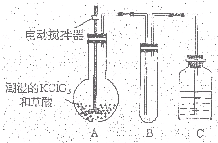 �������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ-59�棬�е�Ϊ11.0�棬������ˮ��ClO2���Կ����������ᣨHClO2�������ᣨHClO3���Ļ����������ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶã�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2�����г�������ʡ�ԣ����ش����⣺
�������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ-59�棬�е�Ϊ11.0�棬������ˮ��ClO2���Կ����������ᣨHClO2�������ᣨHClO3���Ļ����������ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶã�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2�����г�������ʡ�ԣ����ش����⣺