��Ŀ����
����Ŀ����ѧ�ͻ���������ʳƷӪ��������Ӧ�õ�������أ�
(1)���λ�����Ⱦ��������̬�����ѳ�Ϊȫ����Ĺ�ʶ��
�����и����У���ɿ�����������ʱ����Ҫ������ (����ĸ)��
a��������̼��Ũ�� b�����������Ũ�� c��������������Ũ��
��ú���������Ǹ�Ч����������ú̿����Ҫ;����д���Ʊ�ˮú���Ļ�ѧ����ʽ ��
�����������в����ڸ��ƻ����������� (����ĸ)��
a�����Ͼɵ�ؽ���������
b��������÷��ܵ�����Դ
c����������װβ��������װ��
�ܹ�ҵ��ˮ�账����������ŷţ����ֳ��ų��ķ�ˮ��Ҫ�Ǻ���������Է�ˮ�������˷�ˮ�ɲ��õķ����� (����ĸ)��
a��������ԭ�� b���кͷ� c��������
�ݾ��һ�����Ⱦ����Σ�����ǵĽ�����װ���ϻӷ��� �� �dz����ľ��һ�����Ⱦ�������ʯ���е������� �ԣ�Ҳ�dz����ľ��һ�����Ⱦ�
(2)Ӫ��ƽ�⡢������ҩ�DZ�֤���彡����������������Ҫ;����
�����⡢�ơ���������Ԫ���У��������������������Ԫ�ص��� ��(��Ԫ�ط���)
�����������У����п���������Ч���� (����ĸ)��
a�������� b����˹ƥ�� c����ù��
����ͼΪijƷ�Ƽ�����ǩ��һ���֣�������ˮ�����ɰ������������ ��������ɫ������ �����ڷ��������� ��

(3)������������ᷢչ�����ʻ��������Ͽ�ѧ�ķ�չ�벻����ѧ��
��ˮ�ࡢ�������մɶ��Ǵ�ͳ�Ĺ����β��ϣ�����ˮ��Ͳ������õ���ԭ���� �� SiC��һ�����͵��մɣ���ҵ����ʯӢɰ�뽹̿����������������SiCͬʱ����CO�����Ʊ���Ӧ�Ļ�ѧ����ʽΪ
�ڸ�����Ʒ�ڳ�ʪ�Ŀ����з��� (���ѧ���绯ѧ��)��ʴ����ɸ�����ʴ����Ҫԭ��д����������������ʴʱ��������Ӧ
������������̥����Ҫԭ�ϣ���Ȼ��ͨ�� (����������������ϻ���)��ʩ��������ǿ�ȡ����Ժͻ�ѧ�ȶ��Ե���
���𰸡�
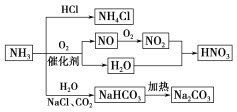
(1)��a����H2O(g)��C(s)![]() H2(g)��CO(g)����a����b���ݼ�ȩ������������
H2(g)��CO(g)����a����b���ݼ�ȩ������������
(2)��Fe����c���ۼ��������������������
(3)��ʯ��ʯSiO2+3C![]() SiC+2CO�����ڵ绯ѧ2H2O+O2+4e-=4OH-������
SiC+2CO�����ڵ绯ѧ2H2O+O2+4e-=4OH-������
��������
���������(1)�ٿ��������������Ҫ���ݰ�����������Ⱦָ������Ҫ��Ⱦ��(����������������������������������)�������������𡢿�������״���ȣ�����Ҫ��������̼��Ũ�ȣ���a��ȷ���ʴ�Ϊ��a��
��ˮ�뽹̿�ڸ����·�Ӧ�Ƶ�ˮú������ˮ�뽹̿�ڸ����·�Ӧ����һ����̼����������ѧ����ʽΪH2O(g)��C(s) ![]() H2(g)��CO(g)���ʴ�Ϊ��H2O(g)��C(s)
H2(g)��CO(g)���ʴ�Ϊ��H2O(g)��C(s) ![]() H2(g)��CO(g)��
H2(g)��CO(g)��
��a���Ͼɵ���к����ؽ��������������� ����Ⱦˮ��Դ��������Ӧ���մ�������a����b���������̫���ܵ������Դ�������˻�ʯȼ�ϵ�ʹ�ã������ڸ��ƻ�����������b��ȷ��c����������װβ��������װ�ã���������Ⱦ��һ����̼��һ���������ŷţ������ڸ��ƻ�����������c��ȷ���ʴ�Ϊ��a��
�� ��ˮ�к������ᣬ����������ˮ��������Һ�����ԣ�����Ҫ�ڷ�ˮ�м���������ʣ�ʹ�䷢������кͷ�Ӧ�����ͷ�ˮ�����ԣ����Բ��õĴ����������кͷ����ʴ�Ϊ��b��
��װ���ϻӷ�����ȩ�ͱ��dz����ľ��һ�����Ⱦ�������ʯ���е��������������ԣ�Ҳ�dz����ľ��һ�����Ⱦ�����ʴ�Ϊ����ȩ������������
(2)�����⡢�ơ���������Ԫ���У��������������������Ԫ�ص��������ʴ�Ϊ��Fe��
�ڰ������ҩ����˹ƥ�־��н�����ʹ���ã���ù��������ҩ���ʴ�Ϊ��c��
�۸��ݼ�����к��е����ʣ�������ˮ�����ɰ����ᣬ��������ɫ�������������Ƿ��������ʴ�Ϊ������ۣ����ƣ�����������
(3)������������ԭ���Ǵ��ʯ��ʯ��ʯӢ������ˮ���ԭ����ճ����ʯ��ʯ����������ˮ��Ͳ������õ���ԭ����ʯ��ʯ����ɫ������������������������(Co2O3)��Ե�ʣ���ҵ����ʯӢɰ�뽹̿����������������SiCͬʱ����CO������ʽΪ��SiO2+3C H2O(g)��C(s) ![]() H2(g)��CO(g)SiC+2CO�����ʴ�Ϊ��ʯ��ʯ��Co2O3��SiO2+3C H2O(g)��C(s)
H2(g)��CO(g)SiC+2CO�����ʴ�Ϊ��ʯ��ʯ��Co2O3��SiO2+3C H2O(g)��C(s) ![]() H2(g)��CO(g)SiC+2CO����
H2(g)��CO(g)SiC+2CO����
�ڸ����ĸ�ʴ��Ҫ�ǵ绯ѧ��ʴ�е�������ʴ���غ�������ʪ�Ŀ����к�����NaCl����ʣ��������γ�ԭ��أ���Ϊ��������ӿ��˽�����ʴ���ʹ�����ȣ�Ӳ����ǿ�ȸߣ��ʴ�Ϊ���绯ѧ���ߣ�
����Ȼ��ͨ����ʩ������ǿ�Ⱥ͵��ԣ��ʴ�Ϊ������

����Ŀ����25��ʱ��AgCl�İ�ɫ����Һ�У����μ����Ũ�ȵ�KI��Һ��Na2S��Һ���۲쵽���������ȳ��ֻ�ɫ���������ճ��ֺ�ɫ��������֪�й����ʵ��ܶȻ�Ksp(25��)���£����������������
AgCl | AgI | Ag2S | |
Ksp | 1.8��10-10 | 8.51��10-16 | 6.3��10-50 |
A. ����ת����ʵ�ʾ��dz����ܽ�ƽ����ƶ�
B. �ܽ��С�ij�������ת��Ϊ�ܽ�ȸ�С�ij���
C. AgCl�����ڵ����ʵ���Ũ�ȵ�NaCl��CaCl2��Һ�е��ܽ�̶���ͬ
D. 25��ʱ���ڱ���AgCl��AgI��Ag2S��Һ�У�����Ag+��Ũ�Ȳ�ͬ
����Ŀ��ij��ѧʵ��С��Ϊȷ����������ֽ����Ѵ�����������ͼװ�ý���ʵ�飬��Ӧ�������ͷ�Ӧֹͣ��ʱ�������±���
MnO2 ʱ�� H2O2 | 0.1 g | 0.3 g | 0.8 g |
10 mL 1.5% | 223 s | 67 s | 56 s |
10 mL 3.0% | 308 s | 109 s | 98 s |
10 mL 4.5% | 395 s | 149 s | 116 s |

��ش��������⣺
��1��ʢװ˫��ˮ�Ļ�ѧ���������� ��
��2����μ������װ�õ��������� ��
��3����ͬŨ�ȵĹ������⣬��ֽ��������Ŷ����������������Ӷ�_______����ӿ족���������䡱����
��4����ʵ��Ч���͡���ɫ��ѧ���ĽǶȿ��ǣ�˫��ˮ��Ũ����ͬʱ������________g(� 0.1 g���� 0.3 g���� 0.8 g��) �Ķ�������Ϊ�ϼ�ѡ��
��5��ijͬѧ�����������ݺ���Ϊ����������ͬ�����Ķ�������ʱ��˫��ˮ��Ũ��ԽС������Ҫ��ʱ���Խ�٣��༴�䷴Ӧ����Խ�족�Ľ��ۣ�����Ϊ�Ƿ���ȷ___________(���ȷ���� ����ȷ��)��������___________________________��(��ʾ��H2O2���ܶȿ���Ϊ�������)��