��Ŀ����
�����е�3He�Ǻ˾۱䷢��������ȫ���������ʣ������Ϻ�Ԫ����Ҫ��4He��ʽ���ڣ�3He����15 t���ң��������ϵ�3He���������֮�࣬�ɹ�ȫ���翪��500�ꡣ����˵����ȷ����(����)
��3He��4He�Ļ�ѧ���ʻ�����ͬ����3He��4He������ͬ������������3He�˾۱��ǻ�ѧ�仯����3HeҺ���������仯����3He��4He�γɵĵ����о����й��ۼ�����3He��4He�ֱ���ɵ����嵥�ʣ�����ͬ�������ܶ�֮��Ϊ3��4
| A���٢ڢ� | B���٢ܢ� | C���ڢۢ� | D���٢ۢ� |
B
����

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д����л�ѧ�����ģ�ͱ�ʾ����ȷ����
A��������Ϊ14�Ĺ�ԭ�ӣ�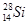 |
B��CH4���ӵı���ģ�ͣ� |
C������ϩ�Ľṹ��ʽ��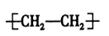 |
| D����������ӵĽṹʽ��H��O��Cl |
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʡ����������������±���ʾ��
| ������ | NH��Na����Mg2�� |
| ������ | OH����NO3����SO42�� |
ȡ�����������ֻ�����������ͬ�������Һ�����������ʵ���Ũ�ȣ�c(��)>c(��)>c(��)���������ʿ�����(����)
��MgSO4����NaOH����(NH4)2SO4����Mg(NO3)2����NH4NO3
A���٢� B���ۢ� C���ۢ� D���٢�
ijѧ����NaHCO3��KHCO3��ɵĻ�������ʵ�飬�����������(ÿ�μ������������ʵ���Ũ�����)�����з�����ȷ����(����)
| ����/mL | 50 | 50 | 50 |
| m(�����)/g | 9.2 | 15.7 | 27.6 |
| V(CO2)(��״��)/L | 2.24 | 3.36 | 3.36 |
A����������ʵ���Ũ��Ϊ3.2 mol��L��1
B���������NaHCO3����������Ϊ54.3%
C��9.2 g�������KHCO3�����ʵ���Ϊ0.05 mol
D��15.7 g�����ǡ����������ȫ��Ӧ
��״����,m1g����A��m2g����B�ķ��������,����˵������ȷ���ǣ� ��
A��1��A���ӵ�������1��B���ӵ�������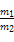 �� �� |
B��ͬ��ͬ�����A��B��������Ϊ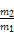 |
C��ͬ��ͬ������A��B�ķ�������Ϊ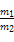 |
D��ͬ��ͬѹ��A��B���ܶȱ�Ϊ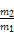 |
Ҫ�������ʵ���Ũ��ԼΪ2 mol��L��1��NaOH��Һ100 mL������IJ�����ȷ����( )
| A����ȡ7.8 g Na2O2���壬����250 mL�ձ��У���100 mL ��Ͳ��ȡ100 mL����ˮ�������ձ��У�ͬʱ���Ͻ����������ܽ� |
| B����ȡ8 g NaOH���壬����100 mL��Ͳ�У��߽����������������ˮ����������ȫ�ܽ��������ˮϡ����100 mL |
| C����ȡ8 g NaOH���壬����100 mL����ƿ�У�������������ˮ��������ƿʹ�����ܽ⣬�ټ���ˮ���̶ȣ��Ǻ�ƿ��������ҡ�� |
| D����100 mL��Ͳ��ȡ40 mL 5 mol��L��1NaOH��Һ������250 mL�ձ��У�������һ��Ͳ��ȡ60 mL����ˮ�����Ͻ����£����������ձ��� |
һ���¶��£�����ͭ���ȷֽ�����CuO��SO2��SO3��O2����֪��SO2��SO3���ܱ���ʯ�Һ�����������Һ���ա�����ͼ��ʾװ�ü�������ͭ��ĩֱ����ȫ�ֽ⡣����ͭ��ĩ����Ϊ10.0 g����ȫ�ֽ��װ�õ������仯��ϵ���±���ʾ��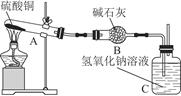
| װ�� | A���Թܣ���ĩ�� | B | C |
| ��Ӧǰ | 42.0 g | 75.0 g | 140.0 g |
| ��Ӧ�� | 37.0 g | 79.5 g | 140.0 g |
��ͨ�����㣬�ƶϳ���ʵ������������ͭ�ֽ�Ļ�ѧ����ʽ�ǣ�������
A��4CuSO4
 4CuO��2SO3����2SO2����O2��
4CuO��2SO3����2SO2����O2�� B. 3CuSO4
 3CuO��SO3����2SO2����O2��
3CuO��SO3����2SO2����O2��C��5CuSO4
 5CuO��SO3����4SO2����2O2��
5CuO��SO3����4SO2����2O2�� D��6CuSO4
 6CuO��4SO3����2SO2����O2��
6CuO��4SO3����2SO2����O2�� ���������ΰ�һ��������Ϻ��ۣ����Ƶû�����X��X����ˮ�ܵ����K����Cr3����SO42��������2.83 g X�е�Cr3��ȫ������ΪCr2O72������Һ�е�Cr2O72���ɺ���KI��Һ��Ӧ���õ�3.84 g I2����Ӧ�����ӷ���ʽΪCr2O72����6I����14H��=2Cr3����3I2��7H2O����������2.83 g X����Һ�У����������BaCl2��Һ���ɵõ�4.66 g��ɫ�������ɴ˿��ƶϳ�X�Ļ�ѧʽΪ(����)
| A��K2SO4��Cr2(SO4)3 | B��2K2SO4��Cr2(SO4)3 |
| C��K2SO4��2Cr2(SO4)3 | D��K2SO4��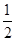 Cr2(SO4)3 Cr2(SO4)3 |