��Ŀ����
ij�ᾧˮ���ﺬ�����������Ӻ�һ�������ӡ���ȡ����������Ϊ45.3g�ĸýᾧˮ����ֱ��Ƴ���Һ��������һ����μ���NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����������ݳ����������д̼�����ζ����ʹʪ��ĺ�ɫʯ����ֽ���������Ⱥƿ��ռ���2.24L�����壨��״����������ɫ�������ٲ�������ʧ����һ����μ���2.0 mol��L-1 Ba��OH��2��Һ����ʼ�������ƣ����������а�ɫ���������ˣ���ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����46.6g��
��ش��������⣺
��1���ýᾧˮ�����к��е�����������
��2����ͨ������ȷ���ýᾧˮ����Ļ�ѧʽ
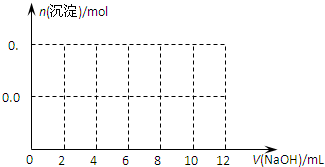
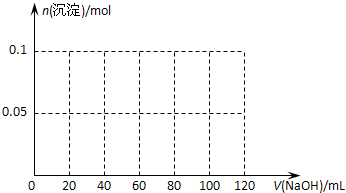
��3����ͨ������ȷ����ֻ����75mL��Ba(OH)2��Һ���õ��ij���������
��1�������д̼�����ζ����ʹʪ��ĺ�ɫʯ����ֽ������˵������NH4+�������2.24L�����ʵ�����0.1mol������ɫ�������ٲ�������ʧ���������������������������Һ�к���Al3+��
��2�����յij���Ҳ�����ᱵ���������ʵ�����46.6g��233g/mol��0.2mol����ѧʽΪNH4Al(SO4)2 ��xH2O�����Ը��������غ��֪���е�ˮ��������45.3g��0.1mol��237g/moll��21.6g�����ʵ�����1.2mol�����Ի�ѧʽΪNH4Al(SO4)2 ��12H2O�� (NH4)2SO4 ��Al2(SO4)3 ��24H2O
��3��75mL��Ba(OH)2�����ʵ�����0.075L��2.0mol/L��0.15mol������OH�������ʵ�����0.3mol�����ε����ʵ�����0.1mol���������ɵij�����0.1mol������������0.15mol�����ᱵ��������0.1mol��78g/mol��0.15mol��233g/mol����42.75g
����

 һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�