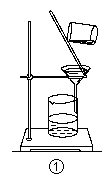
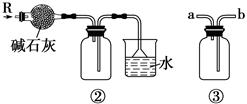
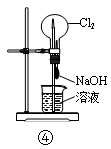
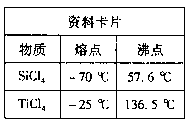
��Ŀ����
ij��ѧʵ���Ҳ����ķ�Һ�к���Fe3����Cu2����Ba2����Cl���������ӣ���������з����Է�Һ���д������Ի��ս������Ʊ��Ȼ������Ȼ������塣

��1������1�к��еĽ��������� ��
��2������ʱ����H2O2��Һ������Ӧ�����ӷ���ʽΪ ��
��3�����������У�������Ϊ�Լ�X���� ������ĸ����
��4���������2ϴ���Ƿ���ȫ�ķ����� ��
��5���Ʊ��Ȼ�������������豣�������������Ŀ���� ��
��6���ɹ���2�õ�����Һ�Ʊ�BaCl2��ʵ���������Ϊ ����ȴ�ᾧ�� ��ϴ�ӡ����

��1������1�к��еĽ��������� ��
��2������ʱ����H2O2��Һ������Ӧ�����ӷ���ʽΪ ��
��3�����������У�������Ϊ�Լ�X���� ������ĸ����
| A��BaCl2 | B��BaCO3 |
| C��NaOH | D��Ba(OH)2 |
��5���Ʊ��Ȼ�������������豣�������������Ŀ���� ��
��6���ɹ���2�õ�����Һ�Ʊ�BaCl2��ʵ���������Ϊ ����ȴ�ᾧ�� ��ϴ�ӡ����
��1������ͭ��2�֣���1�֣�
��2��2Fe2����2H����H2O2��2Fe3����2H2O��2�֣�
��3��BD��2�֣���1�֣�
��4��ȡ���һ��ϴ��Һ����������1��2����������Һ���������ְ�ɫ���ǣ�������ϴ����ȫ������ȡ���һ��ϴ��Һ����������1��2����������Һ���������ְ�ɫ���ǣ�������ϴ����ȫ������2�֣�
��5������Fe3��ˮ�⣨�����𰸾��ɣ���2�֣�
��6������Ũ�� ���ˣ�2�֣���1�֣�
��2��2Fe2����2H����H2O2��2Fe3����2H2O��2�֣�
��3��BD��2�֣���1�֣�
��4��ȡ���һ��ϴ��Һ����������1��2����������Һ���������ְ�ɫ���ǣ�������ϴ����ȫ������ȡ���һ��ϴ��Һ����������1��2����������Һ���������ְ�ɫ���ǣ�������ϴ����ȫ������2�֣�
��5������Fe3��ˮ�⣨�����𰸾��ɣ���2�֣�
��6������Ũ�� ���ˣ�2�֣���1�֣�
�����������1��������м�ܰ�ͭ�û�������ͬʱ����ʣ�࣬����Ϊ����ͭ����2�����������������������Ϊ�����Ӻ��ٳ�����ȥ��˫��ˮ����Fe2������3������X�Լ���Ϊ�˵���pHʹ������������������������Ϊ�˲����������ʿ��Լ���BaCO3��Ba(OH)2�����ʣ���4���������ϴ���Ƿ�ɾ�һ��Ӧȡ���ε���Һ������ܺ��е��������ӣ�һ�������������ӡ������ӵȣ���5���Ȼ�����ˮ��Һ��ˮ�⣬�����������������Fe3��ˮ�⣻��6�����˺�õ�����ҺΪϡ��Һ������Һ�еõ�������Բ�������Ũ�����½ᾧ�ķ�����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ