��Ŀ����
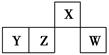
������Ԫ��A��X��D��E��R��Tԭ��������������ԭ�ӽṹ�����������ʾ��
��1��RԪ�������ڱ���λ���� ��������DT�д��ڵĻ�ѧ���� ��
��2��д��E������NaOH��Һ��Ӧ�����ӷ���ʽ ��
��3��1g X2A4 ��ȫȼ�գ��ָ�������ʱ�ų�a kJ��������д��X2A4��ȫȼ�յ��Ȼ�ѧ����ʽ ��
��4��RT4����ˮ�����������ᣬд���÷�Ӧ�Ļ�ѧ����ʽ ��
��5����֪ij�¶���T��ijһԪ�������Ka = 4��0��10-8����һ��Ũ�ȸ����pH=4�������Һ�����ʵ���Ũ��Ϊ ��
| Ԫ�� | �ṹ������ |
| A | A��ԭ�Ӱ뾶��С |
| X | Xԭ�������������Ǵ��������� |
| D | D�Ƕ������н�������ǿ��Ԫ�� |
| E | E������������Ӧˮ������һ�ֳ����������������� |
| R | R��Xͬ���� |
| T | T�ĸ�һ�������ӵĺ�������Ų���Arԭ����ͬ |
��1��RԪ�������ڱ���λ���� ��������DT�д��ڵĻ�ѧ���� ��
��2��д��E������NaOH��Һ��Ӧ�����ӷ���ʽ ��
��3��1g X2A4 ��ȫȼ�գ��ָ�������ʱ�ų�a kJ��������д��X2A4��ȫȼ�յ��Ȼ�ѧ����ʽ ��
��4��RT4����ˮ�����������ᣬд���÷�Ӧ�Ļ�ѧ����ʽ ��
��5����֪ij�¶���T��ijһԪ�������Ka = 4��0��10-8����һ��Ũ�ȸ����pH=4�������Һ�����ʵ���Ũ��Ϊ ��
��1���������ڢ��壨1�֣� ���Ӽ���1�֣�
��2��2Al+2OH?+2H2O = 2AlO2-+3H2�� ��2�֣�
��3��C2H4(g)+3O2(g)=2CO2(g)+2H2O(l) ?H=" -" 28a kJ?mol-1 ��2�֣�
��4��SiCl4+3H2O =H2SiO3��4HCl�� ����SiCl4+4H2O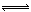 H4SiO4��+4HCl�������Բ��ꡰ������������2�֣�
H4SiO4��+4HCl�������Բ��ꡰ������������2�֣�
��5��0��25mol?L?1 ��2�֣�
��2��2Al+2OH?+2H2O = 2AlO2-+3H2�� ��2�֣�
��3��C2H4(g)+3O2(g)=2CO2(g)+2H2O(l) ?H=" -" 28a kJ?mol-1 ��2�֣�
��4��SiCl4+3H2O =H2SiO3��4HCl�� ����SiCl4+4H2O
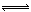 H4SiO4��+4HCl�������Բ��ꡰ������������2�֣�
H4SiO4��+4HCl�������Բ��ꡰ������������2�֣���5��0��25mol?L?1 ��2�֣�
���������A��ԭ�Ӱ뾶��С����AΪHԪ�أ�Xԭ�������������Ǵ�������������XΪCԪ�أ�D�Ƕ������н�������ǿ��Ԫ�أ���DΪNaԪ�أ�E������������Ӧˮ������һ�ֳ������������������EΪAlԪ�أ�R��Xͬ���壬��RΪSiԪ�أ�T�ĸ�һ�������ӵĺ�������Ų���Arԭ����ͬ����TΪClԪ�ء�
��1��RΪSiԪ�أ���Ԫ�����ڱ���λ�ڵ������ڢ��壻������DTΪNaCl���������Ӽ���
��2��EΪAl����NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al+2OH?+2H2O = 2AlO2-+3H2��
��3��X2A4 ΪC2H4��1g C2H4��ȫȼ�գ��ָ�������ʱ�ų�a kJ����������1mol C2H4��28g��ȫȼ�գ��ָ�������ʱ�ų�28a kJ���������ɵ��Ȼ�ѧ����ʽ��C2H4(g)+3O2(g)=2CO2(g)+2H2O(l) ?H=" -" 28a kJ?mol-1
��4��RT4ΪSiCl4��ˮ�����ɵ�������ΪH2SiO3��HCl����H4SiO4��HCl������ˮ�ⷽ��ʽΪ��SiCl4+3H2O =H2SiO3��4HCl�� ����SiCl4+4H2O
 H4SiO4��+4HCl����
H4SiO4��+4HCl������5�������pH=4�������������H+Ũ��Ϊ10-4mol?L?1�����������ʵ���Ũ��ΪC�����ݵ���ƽ�ⳣ���ɵã�Ka =10-4��10-4�£�c-10-4��= 4��0��10-8�����c=0��25mol?L?1

��ϰ��ϵ�д�
�����Ŀ