��Ŀ����
����Ŀ��������Դ�����þ��й���ǰ����
��1�����辭����ѧ�仯���ܴӺ�ˮ�л�õ�������________������ţ�
A��Cl2 B����ˮ C���ռ� D��ʳ��
��2���Ӻ�ˮ����ȡ�����Ҫ��������Ũ���ĺ�ˮ��ͨ�����������������������÷�Ӧ�����ӷ���ʽ��________________________��
��3����ͼ�ǴӺ�ˮ����ȡþ�ļ����̡�
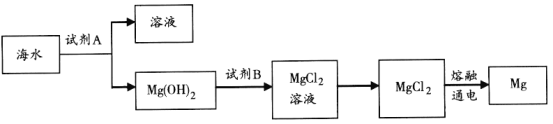
�ٹ�ҵ�ϳ����ڳ���Mg2�����Լ�A��________��תMg��OH��2��ΪMgCl2�����ӷ���ʽ��________________________________��
������ˮMgCl2��ȡMg�Ļ�ѧ����ʽ��________________________��
��4���������и�����I����ʽ���ڵĵ�Ԫ�ء�ʵ������ȡI2��;��������ʾ��
![]()
�����պ������ҽ�ʱ���õ���Ҫ����������________________��
�����ữ����Һ�мӹ���������Һ��д���÷�Ӧ�����ӷ���ʽ_______________________��
��Ӧ�������ټ���CCl4����ȡ���������ã����Թ۲쵽CCl4���________ɫ��
���𰸡���1��BD
��2��Cl2+2Br-=Br2+2Cl-
��3����ʯ����������ƣ�Mg��OH��2+2H+=Mg2++2H2O
��MgCl2��������![]() Cl2��+Mg
Cl2��+Mg
��4��������
��2H++2I-+H2O2�TI2+2H2O
����
��������
�����������1��A����ⱥ���Ȼ�����Һ�õ��ռ���������������������������ڻ�ѧ�仯����A����B���Ѻ�ˮ������ȷ������Եõ���ˮ����B��ȷ��C����ⱥ���Ȼ�����Һ�õ��ռ���������������������������ڻ�ѧ�仯����C����D���Ѻ�ˮ��̫����ɹ������ˮ�ֺ�ʳ�Σ�����Ҫ��ѧ�仯���ܹ��Ӻ�ˮ�л�ã���D��ȷ��
������BD��
��2����������ǿ�����ԣ��ܺ������ӷ����û���Ӧ�����壬���ӷ���ʽΪ��Cl2+2Br-=Br2+2Cl-��
�ʴ�Ϊ��Cl2+2Br-=Br2+2Cl-��
��3���ٹ�ҵ�ϳ���ʯ����������Ƴ���Mg2+��CaO��H2O��Ӧ����Ca��OH��2��Ca��OH��2��þ���ӷ�Ӧ��
��Mg��OH��2��������þ�����ᷴӦ�õ��Ȼ�þ��ˮ����Ӧ�Ļ�ѧ����ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O���������ӷ���ʽΪMg��OH��2+2H+=Mg2++2H2O��
�ʴ�Ϊ��ʯ����������ƣ�Mg��OH��2+2H+=Mg2++2H2O��
������״̬�µ���Ȼ�þұ��þ����ⷴӦ����ʽΪMgCl2��������![]() Cl2��+Mg��
Cl2��+Mg��
�ʴ�Ϊ��MgCl2��������![]() Cl2��+Mg��
Cl2��+Mg��
��4�������չ���ʱ���õ���Ҫ����������������
�ʴ�Ϊ��������
�ڼ��������Ӻ��������������Ϊ����������������ת��Ϊ���ʵ⣬���ӷ���ʽΪ2H++2I-+H2O2�TI2+2H2O��
�ʴ�Ϊ��2H++2I-+H2O2�TI2+2H2O��
�۵��CCl4��Һ����ɫ��
�ʴ�Ϊ������

 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�����Ŀ����ͼΪԪ�����ڱ���һ���֣�����Ԫ�������������ڱ��е�λ�ã���Ҫ��ش��������⡣
�� | IA | 0�� | ||||||
1 | �� | IIA | IIIA | ��A | VA | ��A | VIIA | |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | |||
��1����Ԫ���������У���������ǿ��Ԫ����_____________����Ԫ�ط��ţ���������γɻ������Ԫ����______________����Ԫ�ط��ţ���
��2���õ���ʽ��ʾ������̬�⻯����γɹ���__________________________________��
��3��������������ԭ�Ӱ뾶�ɴ�С��˳����____________________����Ԫ�ط��ţ���
��4����������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������X�ױ����ֽ⡣ijͬѧȡ5֧��С��ͬ���Թܣ�����������ʵ���Ũ�ȵ������X��Һ���ֱ��������ʵ�飬�о����������X�ֽⷴӦ���ʵ�Ӱ�죬ʵ���¼���±���ʾ��
��� | ���� | ���� | ���� | ||
�¶ȣ��� | ���� | ||||
��һ�� | 1 | 40 | FeCl3��Һ | ���ٲ����������� | ��ͬ�����£��¶����ߣ���ѧ��Ӧ���ʼӿ� |
2 | 20 | A | ���������������� | ||
3 | 5 | FeCl3��Һ | ������������������ | ||
�ڶ��� | 4 | t | MnO2 | ���ٲ����������� | |
5 | 20 | �� | ������������������ | ||
����һ��ʵ��Ŀ���ǣ���ͬ�����£�̽��________________�Ը÷�Ӧ���ʵ�Ӱ�졣
ʵ��2�Ĵ���A��___________________��
���ڶ���ʵ���У�ʵ��4���¶�t��_________________��������Ӧ�Ļ�ѧ����ʽ��__________________��
�ڶ���ʵ������ǣ�__________________________________________________��
