��Ŀ����
��ȩ��HCHO�������������ƾ���ǿ��ԭ�ԣ�40%��ȩ��Һ�е�Ϊ96�棬�ӷ���Ϊ̽��������ȩ������Cu(OH)2��Ӧ�IJ�����������о���
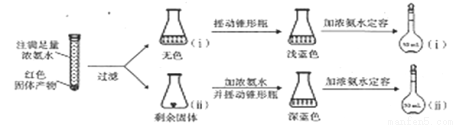
(1)����ͼװ���н���ʵ�飬��a�м���0.5 mol��L-1CuSO4��Һ50mL��5 mol��L-1NaOH��Һ100mL�����ټ���40%�ļ�ȩ��Һ50mL����������a����65��ʱ����20���Ӻ���ȴ�����¡���Ӧ�����й۲쵽����ɫ�������ɣ�����ɺ�ɫ���������������
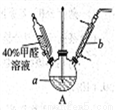
������b��������___________������Ϊ__________��
����˵����ȩ���л�ԭ�Ե�ʵ��������___________��
(2)�������Ϸ�����������Ǹ���Ӧ�����ġ�Ϊȷ����������к�H2����CO����װ��A������ͼ��ʾ��װ�����Ӻ����ʵ�顣
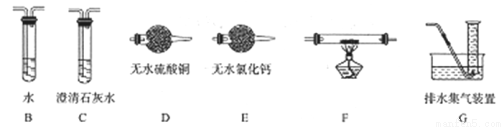
�������ӵĺ���˳��ΪA��B��___��___��___��___G��װ��B��������_________��
(3)��֪��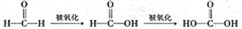 �����ʵ��֤��a�м�ȩ��̼Ԫ��δ��������+4�ۡ�_________________��
�����ʵ��֤��a�м�ȩ��̼Ԫ��δ��������+4�ۡ�_________________��
(4)Ϊ�о���ɫ����������ɣ���������ʵ�飨����ÿ������ַ�Ӧ����
��֪��Cu2O [Cu(NH3)4]+����ɫ��
[Cu(NH3)4]+����ɫ�� [Cu(NH3)4]2+]����ɫ����
[Cu(NH3)4]2+]����ɫ����
��ҡ����ƿ����Ŀ����_____________��
����ƿ���й�����ȫ�ܽ������ɫ��Һ�����ӷ���ʽΪ______________��
�۽�����ƿ���е���Һϡ��100������Һ����ɫ������ƿ��������ɴ˿�֪����������ɼ����ʵ���֮��ԼΪ_____________��
 ��У����ϵ�д�
��У����ϵ�д���ʽ̼��ͭ��Cu2(OH)2CO3��������Ŀ��ﱦʯ��ȸʯ����Ҫ�ɷ֣�Ӧ�ù㷺����������ҵ�������������ͭ������л���ҵ�������л��ϳɴ����ȵȣ�ij��ѧС��Ϊ��̽����ʽ̼��ͭ��������������ʵ�Ӱ�죬���������ʵ�飺
���ԭ����ȡһ�������̼������Һ��0.5mol/L)��100mL�ձ��У����м��ȣ����º�����ͭ��Һ��5.00mL. 0.5mol/L)�ڲ��Ͻ�������һ���ٶ���μ��뵽����̼������Һ�У���Ӧ��ƽ���ֹ����ѹ���ˣ�ϴ�ӣ���ɣ����õ����ղ�Ʒ��ͬʱ������ų���
��1����Ӧԭ��Ϊ��________________��
��2��̽����Ӧԭ�������ȶԷ�Ӧ�����Ӱ�졣
�û�ѧС��������ṩ�Լ���֭����ʵ����˵����Ӧԭ�������Բ�Ʒ��Ӱ��
�ṩ�Լ���0.5mol/LNa2CO3��Һ��0.5mol/LCuSO4��Һ��
������д�±��Ŀհ״���
�� | �� | �� | �� | |
����ͭ��Һ���/mL | 2.0 | 2.0 | 2.0 | 2.0 |
̼������Һ���/mL | 1.6 | 2.0 | 2.8 | |
Na2CO3/CuSO4mol/��) | 0.8 | 1 | 1.4 |
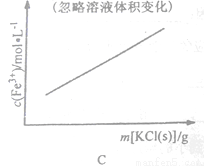
��ͨ��ʵ�黭ͼ��֪������ֵΪ______����ʽ̼��ͭ������á�
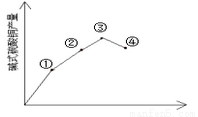
��3����Ӧ�¶ȶԲ��ʵ�Ӱ��
����֧�Թ��и�����2.0mL0.5 mol/LCuSO4��Һ��ȡ��֧�Թܸ�����������ʵ��õ��ĺ���������0.5 mol /L Na2CO3��Һ�����������Թ��и�ȡһ֧�����Ƿքe�������¡�30�桢50�桢100��Ļ����������Ӻ�CuSO4��Һ����Na2CO3��Һ�����۲������֣�������ɫ�ֱ�Ϊ��ɫ��������ɫ����������ɫ��������ɫ�д��к�ɫ������ʵ����Ҳ����ͼ��ʾ����˼����Ϊʲô�¶ȹ��{���ʷ����½�_______����ʵ����ȷ�ʽΪ____________��
��4���������Ƶõļ�ʽ̼��ͭ������������
����ȷ������0.5g������Ʒ����300mL����ƿ�У�����5mL���ᣬ����ʹ���ܽ⣬����l00mLˮ����ϡ�͡�����2.5gKI��ϣ�����5���Ӻ������ε�����Һ����0.1mol/L�������������Һ���еζ�����_______ʱ����ζ����յ㡣
��Ӧ��ԭ����2Cu2++4I- =2CuI+I2��I2+2S2O32-=2I-+S4O62-
��0.lmol/L���������40 mL����ô������ͭ�������ٷ���Ϊ________������Һ�ĵζ���Ϊ_______��������������Һ�൱�ڱ������ʵ���������λ��g/mL��mg/mL)(������λ��Ч���֣���
��10L�����ܱ������г���X(g)��Y(g)��������ӦX(g)��Y(g) M(g)��N(g)
M(g)��N(g)
ʵ���� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
n(X) | n(Y) | n(M) | ||
�� | 700 | 0.40 | 0.10 | 0.090 |
�� | 800 | 0.10 | 0.40 | 0.080 |
�� | 800 | 0.20 | 0.20 | a |
�� | 800 | 0.10 | 0.10 | b |
����˵����ȷ���ǡ�(����)
A. ʵ����У���5minʱ���n(M)��0.050mol����0��5minʱ���ڣ���N��ʾ��ƽ����Ӧ���ʦ�(N)��1.0��10��2mol/(L��min)
B. ʵ����У��÷�Ӧ��ƽ�ⳣ��K��2.0
C. ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ50%
D. ʵ����У��ﵽƽ��ʱ��b<0.05


 6H2(g) + 2CO2(g)�� ��H>0
6H2(g) + 2CO2(g)�� ��H>0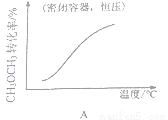


 ������˵���������
������˵���������