��Ŀ����
��10L�����ܱ������г���X(g)��Y(g)��������ӦX(g)��Y(g) M(g)��N(g)
M(g)��N(g)
ʵ���� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
n(X) | n(Y) | n(M) | ||
�� | 700 | 0.40 | 0.10 | 0.090 |
�� | 800 | 0.10 | 0.40 | 0.080 |
�� | 800 | 0.20 | 0.20 | a |
�� | 800 | 0.10 | 0.10 | b |
����˵����ȷ���ǡ�(����)
A. ʵ����У���5minʱ���n(M)��0.050mol����0��5minʱ���ڣ���N��ʾ��ƽ����Ӧ���ʦ�(N)��1.0��10��2mol/(L��min)
B. ʵ����У��÷�Ӧ��ƽ�ⳣ��K��2.0
C. ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ50%
D. ʵ����У��ﵽƽ��ʱ��b<0.05
��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ


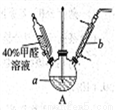
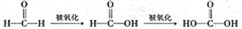 �����ʵ��֤��a�м�ȩ��̼Ԫ��δ��������+4�ۡ�_________________��
�����ʵ��֤��a�м�ȩ��̼Ԫ��δ��������+4�ۡ�_________________��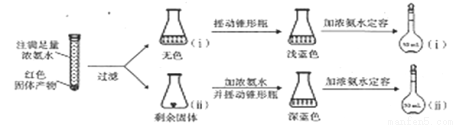
 [Cu(NH3)4]+����ɫ��
[Cu(NH3)4]+����ɫ�� [Cu(NH3)4]2+]����ɫ����
[Cu(NH3)4]2+]����ɫ����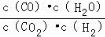 ������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ���ǣ� ��
������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ���ǣ� �� CO2+H2
CO2+H2