��Ŀ����
��12�֣���ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ��
��1������˵���У�����ʵ�鰲ȫ�淶Ҫ����� ���������ĸ����
�� ���������ζʱ��Ӧ������ƿ������ɿ����ʹ����������Ʈ���ǿ�
�� ��ʵ������ͭ��Ũ���ᷴӦ�Ʊ�����������ʵ��ʱ��Ӧ����ͨ����н���
����H2��ԭCuOʵ��ʱ���ȼ���CuO�����£�Ȼ������ͨ��H2ʹ��Ӧ����
��ʵ���е��������Ĺ�������ʣ��ʱ����ֽ�����ú����������Ͱ��
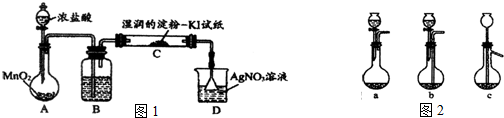
��2��ijѧϰС������ͼ��ʾʵ��װ�ã��г���������ȥ��̽��ͭ˿�����Ũ����ķ�Ӧ�����ֲ�������ʣ�װ���е�ͭ˿���ʵ����³鶯����
�� ����Ӧ����һ��ʱ�����ʹ��ӦѸ��ֹͣ���ɲ�ȡ�Ĵ�ʩΪ ��
�� ��װ�â������������ɵ������������ʣ���װ�âڵ��Թ��м�����Լ�ΪƷ����Һ��ͨ�����������Ϊ ��������֤�������������� �ԡ�
�� װ�â���ʢ��ij���ʵ�ˮ��Һ�����������������Ļ�ԭ�ԣ���õ��ʵ�ˮ��ҺΪ
����Ӧ�Ļ�ѧ����ʽΪ ��
�� ʵ�����Ҫ��ͼ�ٴ��Թ��е�Һ����ˮ��ϣ��Թ۲���ɫ������ڵ����ӣ��Ӱ�ȫ�Ƕȿ��ǣ���ϵķ���Ӧ���� ��
�� ʵ���У����������������������Ӷ���õ�����£�ͬѧ�����ŵ��˽�ǿ�Ĵ̼�����ζ��˵����ʵ�������װ�������ȱ�ݣ�����ɿ�����Ⱦ���Ľ���ʵ��װ�õķ����� ��
����12�֣���1����2�֣��٢�
��2����10�֣������ϣ����⣩�鶯ͭ˿��ʹͭ˿����Ũ���ᣨ1�֣���
��Ʒ����Һ��ɫ��1�֣���Ư���ԣ�1�֣���
����ˮ������ˮ����ˮ����1�֣���Cl2 + SO2 + 2H2O =" 2HCl" + H2SO4��2�֣���
�ܽ����д��Թ�����ȴ��IJ���Һ�����ձ��ڱڻ���ע��ʢ������ˮ���ձ��У������Ͻ��裨2�֣�;
�� ��װ�â۴���һ��װ��NaOH��Һ��ϴ��ƿ���������ж�β����2�֣�
���������������1����Щ������ж��ԣ�����NO2������Ӧ����ͨ����н��У�ѡ��٢���ȷ�������ǿ�ȼ�����壬Ӧ���Ƚ��д��ȵļ��飬�۲���ȷ���������ƾ���ǿ�����ԣ��Ҽ���ˮ��Ӧ�����������ƺ��������������ⶪ�����ܴ���ѡ�٢ڡ�
��2���ٸ���װ�õ��ص��֪����ʹ��ӦѸ��ֹͣ���ɲ�ȡ�Ĵ�ʩ�����ϣ����⣩�鶯ͭ˿��ʹͭ˿����Ũ���ἴ�ɡ�
��ͭ��Ũ�����ڼ��ȵ������£�������Ӧ����SO2��SO2����Ư���ԣ���ʹƷ����Һ��ɫ��
��SO2���л�ԭ�ԣ�����ˮ����ˮ�Լ���ˮ���������ԣ�SO2��ʹ������ɫ�����Լ���SO2�Ļ�ԭ��ʱ������������ˮ������ˮ����ˮ����
�����ڴ��Թ��л���Ũ���ᣬ��Ũ��������ˮ��ų��������ȣ�������ȷ�������ǽ����д��Թ�����ȴ��IJ���Һ�����ձ��ڱڻ���ע��ʢ������ˮ���ձ��У������Ͻ��衣
������SO2���ڴ�����Ⱦ����Ա�����β������װ�ã�����װ�â۴���һ��װ��NaOH��Һ��ϴ��ƿ���������ж�β����
���㣺����ʵ�鰲ȫ������ͭ��Ũ���ᷴӦ���й��жϡ�SO2�����ʡ������Լ�β��������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬���������߿���������ǿ��ѧ�����ѵ÷֡�������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

 ��У����ϵ�д�
��У����ϵ�д�| A����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ | B���Ӻ�ۿ���������ʱ������Ծ�ֹ״̬�������ۿ������������˶��� | C�����÷���ķ������ɸ����������������Ԥ�����ʵ����� | D����������������ɵģ����ʶ����ɷ��Ӻ�ԭ����ɵ� |